Translate this page into:
Efficacy and safety of glucagon-like peptide-1 receptor agonists in Parkinson’s disease: A systematic review and meta-analysis
*Corresponding author: Alok Singh, Department of Pharmacology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India. draloksingh@aiimsraipur.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Singh MP, Balasundaram MK, Singh A. Efficacy and safety of glucagon-like peptide-1 receptor agonists in Parkinson’s disease: A systematic review and meta-analysis. J Neurosci Rural Pract. doi: 10.25259/JNRP_393_2024
Abstract
Objectives
Parkinson’s disease (PD) is a progressive neurodegenerative disorder causing motor as well as non-motor symptoms due to dopaminergic neuron degeneration. Type 2 diabetes mellitus (T2DM) is linked to an increased risk of PD, and glucagon-like peptide-1 (GLP-1) receptor agonists, used in T2DM management, may offer neuroprotective effects. This meta-analysis evaluates the efficacy and safety of GLP-1 receptor agonists in PD. The aim of the study is to systematically evaluate GLP-1 receptor agonists’ efficacy and safety in managing PD, focusing on motor and non-motor symptoms, disease progression, and safety.
Materials and Methods
We conducted the systematic review and meta-analysis following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, searching Cochrane CENTRAL, Medline, Embase, and ClinicalTrials.gov up to June 2024. Randomized controlled trials (RCTs) comparing GLP-1 receptor agonists (exenatide, liraglutide, lixisenatide) with placebo or standard of care were included. Outcomes assessed were Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) III, II, IV, Parkinson’s Disease Questionnaire (PDQ)-39, adverse events (AEs), serious adverse events (SAEs), and weight loss. Data were analyzed using Review Manager 5.4, with the risk of bias assessed using Cochrane risk-of-bias tool 2.
Results
Five RCTs with 570 PD patients were included. GLP-1 receptor agonists showed better improvement in MDS-UPDRS III (mean difference [MD] = −2.00; 95% confidence interval [CI] = −4.23–0.23; P = 0.08). No significant differences were observed in MDS-UPDRS II (MD = −1.65; 95% CI = −3.39–0.10; P = 0.06), MDS-UPDRS IV (MD = −0.24; 95% CI = −0.91–0.43; P = 0.48), or PDQ-39 (MD = −1.68; 95% CI = −4.55–1.18; P = 0.25). GLP-1 receptor agonists were associated with significant weight loss (risk ratios = 2.49; 95% CI = 1.20–5.16; P = 0.01). No significant differences were found in other AEs or SAEs. Evidence quality was low to very low.
Conclusions
GLP-1 receptor agonists may improve motor symptoms and cause weight loss in PD. Further research is needed to confirm their efficacy and safety.
Keywords
Glucagon-like peptide-1 receptor agonists
Motor symptoms
Parkinson’s disease
Weight loss
INTRODUCTION
Parkinson’s disease (PD) is a common, progressive neurodegenerative disorder characterized by the deterioration of dopaminergic neurons in the nigrostriatal pathway, which plays a vital role in voluntary movement control.[1] The clinical presentation includes characteristic motor symptoms such as tremors, bradykinesia, rigidity, and postural instability, alongside non-motor manifestations including cognitive decline, mood disorders, sleep disturbances, and autonomic dysfunction. Contemporary therapeutic approaches primarily revolve around dopaminergic replacement strategies to mitigate symptoms, without addressing the underlying disease progression.[2,3]
Epidemiological investigations have identified an association between type 2 diabetes mellitus (T2DM) and increased PD risk. This relationship may be attributed to shared pathological mechanisms involving α-synuclein aggregation and insulin resistance.[4,5] Interestingly, diabetic patients receiving glucagon-like peptide-1 targeted therapies demonstrate lower PD prevalence compared to those on alternative antidiabetic medications.[5,6] This clinical observation is reinforced by preclinical evidence indicating that insulin resistance and hyperglycemia can accelerate α-synuclein accumulation and dopaminergic neurodegeneration, resembling PD pathology.[7]
Glucagon-like peptide-1 (GLP-1) receptor agonists are established therapeutic agents for T2DM management and enhance glucose-dependent insulin secretion while suppressing glucagon release, thereby effectively regulating glycemic control.[8] These compounds possess the ability to penetrate the blood-brain barrier and exhibit neuroprotective properties. Pre-clinical investigations reveal their capacity to shield neurons from cytokine-induced apoptosis, enhance neurogenesis, and attenuate neuroinflammation – processes central to PD pathophysiology.[9,10]
The neuroprotective mechanism of GLP-1 receptor agonists involves the activation of neural GLP-1 receptors, triggering signaling cascades that upregulate anti-apoptotic proteins while downregulating pro-apoptotic factors, thereby preventing neuronal death.[11] In addition, these agents mitigate chronic neuroinflammation by reducing pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-1 beta, thus limiting neuronal injury.[9,12] GLP-1 receptor agonists also stimulate neural progenitor cell proliferation and differentiation in critical regions including the hippocampus and substantia nigra, potentially facilitating neuronal replacement and network restoration.[13,14] This regenerative capacity could translate to improvements in both cognitive and motor function in PD patients.
Addressing mitochondrial dysfunction, a hallmark of PD pathology leading to compromised energy production and oxidative stress, GLP-1 receptor agonists enhance mitochondrial performance by optimizing electron transport chain efficiency and diminishing reactive oxygen species generation.[15,16] Furthermore, these compounds facilitate the reduction of α-synuclein aggregation and promote its clearance through autophagy enhancement, enabling the removal of damaged proteins and cellular components.[15,17]
Various GLP-1 receptor agonists including exenatide, liraglutide, and lixisenatide are currently under investigation as potential PD therapeutics.[18] Exenatide has yielded promising outcomes in preliminary clinical trials, demonstrating a reduction in motor symptoms and potential disease-modifying effects.[19,20] Pre-clinical studies with liraglutide and lixisenatide have shown decreased α-synuclein aggregation and improved motor function.[21,22]
Despite these encouraging preliminary findings, significant challenges remain in the clinical development of GLP-1 receptor agonists for PD. These include establishing long-term safety profiles, determining optimal neurological dosing regimens, and addressing potential variability in blood-brain barrier penetration among different agents.
Considering the therapeutic potential of GLP-1 agonists, a thorough evaluation of their efficacy and safety profile is imperative. This meta-analysis aims to systematically examine the evidence supporting GLP-1 agonists for PD treatment, with particular focus on their therapeutic efficacy in ameliorating motor and non-motor symptoms, disease-modifying potential, and safety considerations. Through comprehensive data synthesis, we seek to provide insights to inform clinical practice and direct future research in this promising therapeutic avenue for PD management.
METHODS
The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[23] The study protocol was registered with PROSPERO (CRD42024564860). The PRISMA checklist is attached as Supplementary File S1.
Criteria for including studies
We included randomized controlled trials (RCTs) that focused on adults aged 18 years or older diagnosed with PD based on established clinical criteria, i.e., the UK PD Society Brain Bank criteria or equivalent. The interventions considered were treatments involving GLP-1 receptor agonists (exenatide, liraglutide, and lixisenatide) with/without standard of care (SoC) for PD, compared to placebo with/without SoC. To be eligible, studies had to report primary outcomes related to changes in motor and non-motor functions/symptoms, adverse effects, and biomarkers of disease progression. In addition, only studies with a minimum follow-up period of 12 weeks and published in English were included. Exclusion criteria comprised non-randomized studies, observational studies, case reports, case series, cross-over trials, and review articles. Studies that combined GLP-1 receptor agonists with other investigational drugs or treatments not approved for PD, or those that did not report on the primary or secondary outcomes of interest, were also excluded. Furthermore, studies with a follow-up period of <12 weeks, as well as abstracts, conference proceedings, theses, dissertations, and unpublished data, were not considered. The search strategy is presented in Supplementary File S2.
Types of interventions
Eligible studies administered GLP-1 receptor agonists, irrespective of dosage or duration. Participants in both experimental and control groups could receive additional treatments alongside standard PD therapy. We compared GLP-1 receptor agonists versus standard PD treatment/placebo/no treatment.
Outcome measures
The outcomes included in this meta-analysis are mean change in Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) III at the end of the study (EOS), mean change in MDS-UPDRS II at EOS, mean change in MDS-UPDRS IV at EOS, mean change in Parkinson’s Disease Questionnaire (PDQ)-39 at EOS, proportion of patients experiencing any adverse event (AE), proportion of patients with serious adverse events (SAEs), and proportion of patients with weight loss.
Search methods for identification of studies
We conducted electronic searches from inception to June 2024 in databases including the Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase, and ClinicalTrials.gov. Conference abstracts and clinical trial listings were also reviewed for missing data (if any).
Data collection and analysis
We (MPS, MKB) screened titles and abstracts for inclusion, removing duplicates and independently assessing full-text reports of potentially relevant studies. Disagreements were resolved through consensus. Data extraction included publication details, study eligibility criteria, participant characteristics, intervention details, outcome data, and funding sources. Data were managed using Review Manager 5.4.
Assessment of risk of bias in included studies
The assessment of methodological quality was conducted independently by two investigators (AS, MPS) utilizing the revised Cochrane risk-of-bias tool 2 (RoB2). This evaluation encompassed five key domains: Bias arising from the randomization procedure, deviations from planned interventions, missing outcome data, outcome measurement processes, and selective reporting of results.[24] For each study, these domains were systematically evaluated and categorized. The reviewers documented their judgments in comprehensive risk-of-bias tables, accompanied by supporting rationales and relevant extracts from the original publications to substantiate the assessments.
Statistical analysis
Measures of treatment effect
For data analysis, we employed mean differences (MD) for continuous variables and risk ratios (RR) for dichotomous outcomes, with each measure presented alongside their corresponding 95% confidence intervals (CIs). While analyses were performed using both fixed and random-effects models, we prioritized reporting the random-effects model results due to anticipated clinical heterogeneity. To quantify statistical heterogeneity, we utilized Cochrane’s Q test and the I2 statistic, with heterogeneity considered statistically significant at a threshold of P< 0.10.[25] For studies presenting outcomes in formats other than means and standard deviations, we performed appropriate statistical conversions using validated formulae to standardize the data for meta-analysis.[26]
Quality of the evidence
The strength and reliability of evidence for each primary outcome were systematically evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) methodology. Evidence quality was classified into four distinct levels: high, moderate, low, or very low, based on rigorous assessment criteria. Factors warranting the downgrading of evidence quality were explicitly documented. A comprehensive summary of findings tables was generated according to GRADE guidelines,[27] providing a transparent representation of the evidence quality underpinning our conclusions.
RESULTS
The initial search yielded 1126 abstracts from various databases. Automation tools removed duplicate and ineligible articles and a total of 84 articles were assessed for eligibility. Out of 84 articles, 70 were removed as they were reviewed (41), observation studies (10), case reports (04), letters to the editor (06), and non-human studies (09). Out of 14 articles, 5 were included for quantitative analysis [Figure 1].[28-32] The current meta-analysis included five RCTs published from inception to June 2024 which included 570 patients of PD. In all RCTs, the patients were diagnosed with PD by Queen Square Brain bank criteria. In all the RCTs, patients were included if they belonged to Hoehn and Yahr stage <3, which indicates mild bilateral disease with recovery on the pull test.[33] The patients received one GLP-1 agonist or placebo in addition to the SoC. Out of five studies, 3 studies used exenatide as an intervention[28,29,31] while the other two used liraglutide and lixisenatide, respectively.[30,32] The results of one study were not peer-reviewed.[30] Most of the patients in RCTs were male (~69%) and the mean of the patients was 61 years. SoC in all RCTs was primarily based on dopaminergic drugs [Table 1]. As per RoB2 tool, four RCTs had some concerns[28,30-32] [Table 2].
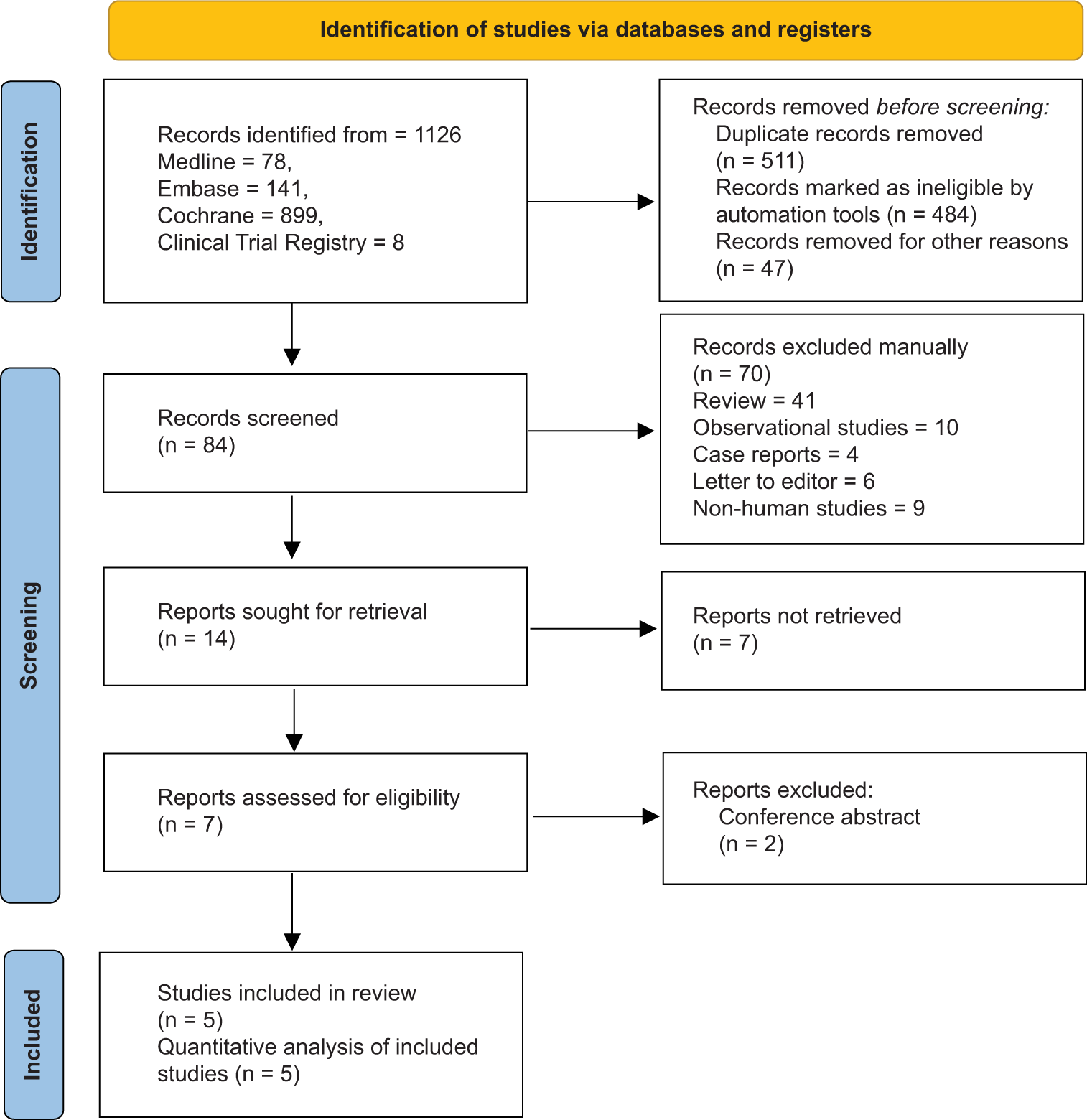
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study selection process.
| Study | Country of origin | Sample Size | Age in years Mean (SD) | Sex (Male: Female) | Study design | Intervention arm | Control arm | Duration |
|---|---|---|---|---|---|---|---|---|
| Aviles-Olmos et al. 2013[28] | United Kingdom | 44 | 60.4 (7.2) | 79: 21 | RCT | SC Exenatide 5-μg self-administered twice-daily injections for 1 month, followed by 10-μg exenatide twice-daily injections for subsequent 11 months+SoC | SoC | 12 months+ 2 month washout period |
| Athauda et al. 2017[29] | United Kingdom | 60 | 59.7 (8.1) | 73.5: 26.5 | RCT | SC Exenatide 2 mg self-administered injection once weekly for 48 weeks for 48 weeks+SoC | Placebo+ SoC * |
48 weeks+ 12 weeks washout period |
| Hogg et al. 2022[30] | USA | 55 | 63.8 (8.1) | 69.9: 30.1 | RCT | SC Liraglutide self-administered injection once daily (1.2 or 1.8, as tolerated) for 52 weeks+SoC | Placebo+SoC | 54 weeks |
| McGarry et al. 2024[31] | USA | 255 | 61.5 (9) | 65.6: 34.4 | RCT | SC NLY01 (2.5 mg and 5.0 mg) once weekly for 36 weeks+SoC | Placebo+SoC | 36 weeks |
| Meissner et al. 2024[32] | France | 156 | 59.7 (8.3) | 59: 41 | RCT | SC lixisenatide at an initial dose of 10 μg/day for 14 days, after which the dose was increased to 20 μg/day for the remain- der of the 12-month period+SoC |
Placebo+SoC | 36 weeks |
RCT: Randomized controlled trial, SC: Subcutaneous, SD: Standard deviation*SoC: Standard of care (generally include levodopa, carbidopa, ropinirole, pramipexole, rasagiline, and rotigotine
| Study | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|
| Avies Olmos et al. 2013 | Some Concerns | Some Concerns | Low Risk | Some Concerns | Low Risk | Some Concerns |
| Athauda et al. 2017 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Hogg et al. 2022 | Some Concerns | Some Concerns | Low Risk | Low Risk | Low Risk | Some Concerns |
| McGarry et al. 2024 | Some Concerns | Low Risk | Low Risk | Low Risk | Low Risk | Some Concerns |
| Meissner’s et al. 2024 | Some Concerns | Low Risk | Low Risk | Low Risk | Low Risk | Some Concerns |
D1: Bias arising from the randomization process. D2: Bias due to deviations from intended interventions. D3: Bias due to missing outcome data. D4: Bias in measurement of the outcome. D5: Bias in selection of the reported result
Efficacy endpoints
All the RCTs had two arms, i.e., SoC and GLP-1 agonist and SoC and placebo. Review authors assessed publication bias by analyzing funnel plots. All funnel plots and forest plots are presented as Supplementary Files S3 and S4, respectively. For the efficacy endpoint, i.e., mean change in UPDRS III at EOS, the funnel plot was symmetrical suggesting no publication bias. Review authors observed significant heterogeneity (I2 = 67%, P= 0.02). The change in UPDRS-III was more in the GLP-1 agonist group indicating more improvement in symptoms of PD compared to the placebo (MD = −2.00; 95% CI = −4.23– 0.23; P = 0.08). In another endpoint that is the mean change in UPDRS-II at EOS, the funnel plot was symmetrical suggesting no publication bias. Significant heterogeneity was noted (I2 = 85%, P < 0.0001). More change in score was observed in the GLP-1 agonist group compared to placebo (better symptoms improvement) and was not statistically significant (MD = −1.65; 95% CI = −3.39–0.10; P = 0.06).
In another endpoint, i.e., mean change in UPDRS IV at EOS, the funnel plot suggested no publication bias, and mild heterogeneity was noted, i.e., I2 = 26%, P = 0.25. Slightly better improvement in PD symptoms was noted in the GLP-1 agonist group compared to placebo (MD = −0.24; 95% CI = −0.91–0.43; P = 0.48). Similarly, for the endpoint mean change in PDQ-39 at EOS, no publication bias was detected and significant heterogeneity was noted (I2 = 69%, P = 0.01). The GLP-1 agonist group showed slightly better symptom reduction than the placebo, which was statistically not significant (MD = −1.68; 95% CI = −4.55–1.18; P = 0.25) The estimates of different endpoints did not change even after applying fixed-effect model except for mean change in UPDRS III at EOS which became statistically significant (MD = −1.90; 95% CI = −3.09–−0.72; P = 0.002).
Safety endpoints
A greater number of patients experienced any AE in the GLP-1 agonist group than placebo (89.3% vs. 79.8%) and was statistically not significant; RR = 1.11; 95% CI = 0.98– 1.26; P = 0.10. The funnel plot was symmetrical and was not suggestive of publication bias. Moderate heterogeneity was observed (I2 = 54%, P = 0.11). The proportion of patients with SAE was higher in the GLP-1 agonist group compared to placebo (11.7% vs. 7.9%) but was not statistically significant; RR = 1.46; 95% CI = 0.73–2.93; P = 0.28. The funnel plot was symmetrical and was not suggestive of publication bias, and the review authors did not observe any heterogeneity (I2 = 0%, P = 0.76). A greater number of patients with weight loss were observed in the GLP-1 agonist group compared to placebo (24.3% vs. 13.1%) and it was statistically significant; RR = 2.49; 95% CI = 1.20–5.16; P = 0.01. The funnel plot was asymmetrical and publication was suggested with significant heterogeneity (I2 = 72%, P = 0.007). The safety estimates were similar after applying the fixed-effect model except in the case of the proportion of patients who experienced any AE that turned significant (RR = 1.10; 95% CI = 1.01–1.19; P = 0.02).
As per the GRADE approach, the overall certainty of the evidence was categorized as very low for multiple primary outcomes, including mean changes in UPDRS III at the EOS, MDS-UPDRS II at EOS, PDQ-39 at EOS, and the proportion of patients experiencing weight loss. The certainty of the evidence was assessed as low for mean change in UPDRS IV at EOS, the proportion of patients reporting any AE, and the proportion of patients with SAE. The comprehensive assessment details and specific estimates for each outcome measure are presented in Table 3.
| Certainty assessment | ||||||
| Participants (studies) Follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence |
| Change in UPDRS-III at EOS | ||||||
| 565 (5 RCTs) |
Not serious | Very seriousa | Not serious | Seriousb | None | ⨁◯◯◯ Very low |
| Change in UPDRS-II at EOS | ||||||
| 565 (5 RCTs) |
Not serious | Very seriousc | Not serious | Seriousb | None | ⨁◯◯◯ Very low |
| Change in UPDRS-IV at EOS | ||||||
| 311 (4 RCTs) |
Not serious | Seriousd | Not serious | Seriousb | None | ⨁⨁◯◯ Low |
| Change in PDQ-39 at EOS. | ||||||
| 565 (5 RCTs) |
Not serious | Very seriouse | Not serious | Seriousb | None | ⨁◯◯◯ Very low |
| Incidence of any AE | ||||||
| 473 (3 RCTs) |
Not serious | Seriousf | Not serious | Seriousb | None | ⨁⨁◯◯ Low |
| Incidence of serious AE | ||||||
| 323 (4 RCTs) |
Not serious | Seriousg | Not serious | Seriousb | None | ⨁⨁◯◯ Low |
| Proportion of patients having weight loss | ||||||
| 577 (5 RCTs) |
Not serious | Very serioush | Not serious | Seriousi | Publication bias strongly suspectedj | ⨁◯◯◯ Very low |
| Participants (studies) Follow-up | Summary of findings | |||||
| Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | ||||
| With placebo | With Change in UPDRS-III at EOS | Risk with placebo | Risk difference with Change in UPDRS-III at EOS | |||
| Change in UPDRS-III at EOS | ||||||
| 565 (5 RCTs) |
230 | 335 | - | 230 | MD 2 lower (4.23 lower to 0.23 higher) |
|
| Change in UPDRS-II at EOS | ||||||
| 565 (5 RCTs) |
230 | 335 | - | 230 | MD 1.65 lower (3.39 lower to 0.1 higher) |
|
| Change in UPDRS-IV at EOS | ||||||
| 311 (4 RCTs) |
146 | 165 | - | 146 | MD 0.24 lower (0.91 lower to 0.43 higher) |
|
| Change in PDQ-39 at EOS. | ||||||
| 565 (5 RCTs) |
230 | 335 | - | 230 | MD 1.68 lower (4.55 lower to 1.18 higher) |
|
| Incidence of any AE | ||||||
| 473 (3 RCTs) |
146/183 (79.8%) | 259/290 (89.3%) | RR 1.11 (0.98–1.26) |
146/183 (79.8%) | 88 more/1,000 (from 16 fewer to 207 more) |
|
| Incidence of serious AE | ||||||
| 323 (4 RCTs) |
12/152 (7.9%) | 20/171 (11.7%) | RR 1.46 (0.73–2.93) |
12/152 (7.9%) | 36 more/1,000 (from 21 fewer to 152 more) |
|
| Proportion of patients having weight loss | ||||||
| 577 (5 RCTs) |
31/236 (13.1%) | 83/341 (24.3%) | RR 2.49 (1.20–5.16) |
31/236 (13.1%) | 196 more/1,000 (from 26 more to 546 more) |
|
UPDRS: Unified Parkinson’s disease rating scale, EOS: End of the study, RCTs: Randomized controlled trials, PDQ: Parkinson’s disease questionnaire, AE: adverse event, CI: Confidence interval, MD: Mean difference, RR: Risk ratio. aSignificant Heterogeneity (I2=67%, P=0.02), bConfidence interval wide, crossing the threshold, cSignificant Heterogeneity (I2=85%, P<0.0001, dMild Heterogeneity Observed (I2=26%), eSignificant Heterogeneity (I2=69%, individual estimates differ among studies), fModerate heterogeneity observed, I2=54%, P=0.11, gIndividual estimates variable among studies, hSignificant heterogeneity I2=72%, P=0.007, individual estimates differ among studies, iVery wide confidence interval, jFunnel plot suggests publication bias, ⨁: Certainty of evidence, ◯: Less certainty
DISCUSSION
This meta-analysis aimed to evaluate the neuroprotective role of GLP-1 agonists in PD. The current SoC for PD centers on dopaminergic drugs that increase dopamine availability in the substantia nigra. However, there are currently no approved neuroprotective agents for PD management.[34] Our analysis encompassed 570 patients with a mean age of 61 years, with a predominance of male participants, aligning with the characteristic epidemiological distribution observed in PD.[34]
Efficacy of GLP-1 receptor agonists
According to our assessment using the RoB2 tool, four of the five included studies demonstrated some concerns regarding the risk of bias.[28,30-32] These concerns primarily stemmed from inadequate information about allocation concealment and insufficient details regarding protocol deviations, leading to their classification as having “some concerns.”
When comparing GLP-1 receptor agonists to placebo, improvements were observed in UPDRS-III, UPDRS-II, and UPDRS-IV scores; however, none of these changes reached statistical significance. These scales, respectively, evaluate motor experiences of daily living, motor examination, and motor complications.[35] The certainty of evidence was assessed as very low for UPDRS-II and III and low for UPDRS-IV [Table 3]. Thus, while GLP-1 receptor agonists demonstrated some improvement in motor symptomatology, these findings align with a previous meta-analysis that noted similar trends but lacked definitive evidence.[18]
Quality of life and AEs
Quality of life assessment using PDQ-39 showed marginally better outcomes in the GLP-1 receptor agonist group, though the evidence was of very low certainty, consistent with previous analyses.[18] The incidence of patients experiencing any AEs and SAEs was higher in the GLP-1 receptor agonist group, although these differences did not reach statistical significance. Notably, the proportion of patients experiencing weight loss was significantly higher in the GLP-1 receptor agonist group, a finding not previously documented in earlier meta-analyses.[18] This observation is supported by other studies reporting weight reduction associated with GLP-1 receptor agonists in type 2 diabetes patients.[36,37] While weight loss may be beneficial for some individuals, it requires careful monitoring in PD patients, for whom maintaining adequate nutritional status is essential.
Contextualizing results and future directions
PD represents a neurodegenerative condition managed symptomatically, with dopaminergic therapy effectiveness typically diminishing over time. Patients eventually develop wearing-off and on-off phenomena, further complicated by levodopa-induced dyskinesias and other adverse effects of dopaminergic medications, including hallucinations, compulsive behaviors, impulse control disorders, and dopamine dysregulation syndrome.[38] Given the progressive nature of neuronal degeneration, halting this process becomes a critical therapeutic goal.
GLP-1 receptor agonists have demonstrated potential in both pre-clinical and clinical investigations for their non-metabolic effects, including enhanced neurogenesis, reduced inflammatory mediators, decreased oxidative stress, and improved mitochondrial function. These properties may contribute to neuroprotection in PD.[18] Despite these promising characteristics, the current evidence from our review remains inconclusive due to study heterogeneity, variations in SoC, utilization of different GLP-1 receptor agonists, and diverse drug administration durations. Long-term data collection is necessary to comprehensively evaluate the neuroprotective potential of GLP-1 receptor agonists, particularly as they are currently approved only for T2DM management.
To our knowledge, this represents the second systematic review and meta-analysis examining GLP-1 receptor agonists in PD, following an initial review conducted in 2020 that included only two studies.[18] Our analysis incorporates five studies, though this number remains relatively limited. In addition, publication bias quantification was not performed in this review.
Strengths and limitations
The strengths of this meta-analysis include its comprehensive search methodology and incorporation of recent RCTs, providing a broader perspective on GLP-1 receptor agonist effects in PD compared to previous reviews. However, limitations include the small number of available trials, variability in study designs, treatment protocols, and follow-up periods, all contributing to the observed heterogeneity. The overall certainty of the evidence was classified as low to very low for several outcomes due to risk-of-bias concerns identified through the RoB tool. The absence of long-term safety and efficacy data further constrains definitive conclusions regarding the extended use of these agents in PD.
Future research
Future investigations should endeavor to overcome these limitations through robust, large-scale trials with extended follow-up periods and standardized protocols for GLP-1 receptor agonist administration. Research should investigate the comparative efficacy of different GLP-1 receptor agonists and elucidate their specific neuroprotective mechanisms in PD pathophysiology. Comprehensive assessment of long-term safety profiles and detailed evaluation of effects on disease progression markers and patient-reported quality of life measures are crucial to accurately determine the therapeutic potential of GLP-1 receptor agonists in PD management.
CONCLUSION
This meta-analysis examined the neuroprotective capabilities of GLP-1 receptor agonists in Parkinson’s disease. Although these agents demonstrated trends toward improvement in motor symptomatology and quality of life metrics compared to placebo, the observed differences failed to achieve statistical significance, and the overall quality of evidence was low. The significantly higher incidence of weight loss among patients receiving GLP-1 receptor agonists necessitates careful consideration in clinical application. Despite encouraging preliminary findings, the current evidence remains inconclusive, limited by the small number of available studies, methodological heterogeneity, and insufficient long-term efficacy and safety data. Additional well-designed clinical trials with larger patient populations are essential to definitively establish the therapeutic efficacy and safety profile of GLP-1 receptor agonists in PD, thereby informing their potential integration into neuroprotective treatment strategies.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required, as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Istradefylline: A novel drug for 'off ' episodes in Parkinson's disease. Drugs Ther Perspect. 2020;36:208-12.
- [CrossRef] [Google Scholar]
- The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11:25-40.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes and the risk of developing Parkinson's disease in Denmark. Diabetes Care. 2011;34:1102-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes medications and risk of Parkinson's disease: A cohort study of patients with diabetes. Brain. 2020;143:3067-76.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of the neuroprotective effect of GLP-1 in a rat model of Parkinson's with pre-existing diabetes. Neurochem Int. 2019;131:104583.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term hyperglycemia aggravates α-synuclein aggregation and dopaminergic neuronal loss in a Parkinson's disease mouse model. Transl Neurodegener. 2022;11:14.
- [CrossRef] [PubMed] [Google Scholar]
- Glucagon-like peptide-1 receptor Agonists In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551568 [Last accessed on 2024 Feb 29]
- [Google Scholar]
- Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550.
- [CrossRef] [PubMed] [Google Scholar]
- GLP-1 Receptor agonists: Beyond their pancreatic effects. Front Endocrinol (Lausanne). 2021;12:721135.
- [CrossRef] [PubMed] [Google Scholar]
- Unlocking the potential: Semaglutide's impact on Alzheimer's and Parkinson's disease in animal models. Curr Issues Mol Biol. 2024;46:5929-49.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroinflammation in Parkinson's disease and its treatment opportunities. Balkan Med J. 2022;39:318-33.
- [CrossRef] [PubMed] [Google Scholar]
- Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res. 2011;89:481-9.
- [CrossRef] [PubMed] [Google Scholar]
- The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: An in-depth review. Front Neurosci. 2022;16:970925.
- [CrossRef] [PubMed] [Google Scholar]
- Glucagon-like peptide-1 receptor agonist ameliorates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity through enhancing mitophagy flux and reducing α-synuclein and oxidative stress. Front Mol Neurosci. 2021;14:697440.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023;66:102849.
- [CrossRef] [PubMed] [Google Scholar]
- Alpha-synuclein in Parkinson's disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023;14:176.
- [CrossRef] [PubMed] [Google Scholar]
- GLP-1 receptor agonists for Parkinson's disease. Cochrane Database Syst Rev. 2020;7:CD012990.
- [CrossRef] [PubMed] [Google Scholar]
- Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr). 2013;35:1621-36.
- [CrossRef] [PubMed] [Google Scholar]
- Exenatide as a potential treatment for patients with Parkinson's disease: First steps into the clinic. Alzheimers Dement. 2014;10(1Suppl):S38-46.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroprotective effects of liraglutide against inflammation through the AMPK/NF-κB pathway in a mouse model of Parkinson's disease. Metab Brain Dis. 2022;37:451-62.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. 2015;303:42-50.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- [CrossRef] [PubMed] [Google Scholar]
- RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- [CrossRef] [PubMed] [Google Scholar]
- The 5 min meta-analysis: Understanding how to read and interpret a forest plot. Eye (Lond). 2022;36:673-5.
- [CrossRef] [PubMed] [Google Scholar]
- Choosing effect measures and computing estimates of effect In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, eds. Cochrane handbook for systematic reviews of interventions version 6.4 Cochrane. Hoboken: John Wiley and Sons; 2023. Ch. 6 Available from: https://www.training.cochrane.org/handbook [Last accessed on 2024 Aug 07]
- [Google Scholar]
- Completing 'summary of findings' tables and grading the certainty of the evidence In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, eds. Cochrane handbook for systematic reviews of interventions version 6.4 Cochrane. Hoboken: John Wiley and Sons Ltd; 2023. Ch. 14 Available from https://www.training.cochrane.org/handbook [Last accessed on 2024 07]
- [Google Scholar]
- Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730-6.
- [CrossRef] [PubMed] [Google Scholar]
- Exenatide once weekly versus placebo in Parkinson's disease: A randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664-75.
- [CrossRef] [PubMed] [Google Scholar]
- A phase II, randomized, double-blinded, placebo-controlled trial of liraglutide in Parkinson's disease. Available from: https://ssrn.com/abstract=4212371 [Last accessed on 2025 Apr 03]
- [Google Scholar]
- Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson's disease: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024;23:37-45.
- [CrossRef] [PubMed] [Google Scholar]
- Trial of lixisenatide in early Parkinson's disease. N Engl J Med. 2024;390:1176-85.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson's disease: A large pragmatic randomised controlled trial (PD REHAB) (Health Technology Assessment. No. 20.63.) Appendix 8, Hoehn and Yahr stages. 2016 Available from: https://www.ncbi.nlm.nih.gov/books/NBK379751 [Last accessed on 2024 Aug 02]
- [CrossRef] [Google Scholar]
- Istradefylline versus opicapone for “off” episodes in Parkinson's disease: A systematic review and meta-analysis. Ann Neurosci. 2021;28:65-73.
- [CrossRef] [PubMed] [Google Scholar]
- Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129-70.
- [CrossRef] [PubMed] [Google Scholar]
- Liraglutide for weight management: A critical review of the evidence. Obes Sci Pract. 2017;3:3-14.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of subcutaneous lixisenatide on weight loss in patients with type 2 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2024;210:111617.
- [CrossRef] [PubMed] [Google Scholar]
- Parkinson’s disease In: Harrison’s principles of internal medicine (20th ed). New York: McGraw Hill Professional; 2018. p. :3120-32.
- [Google Scholar]






