Translate this page into:
Structural magnetic resonance imaging in Parkinson disease with freezing of gait: A systematic review of literature
*Corresponding author: Muhammad Hamdan, Department Neurology, Faculty of Medicine, Airlangga University - Dr Soetomo General Hospital, Surabaya, East Java, Indonesia. luki.hamdan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Suharto AP, Sensusiati AD, Hamdan M, Bastiana DS. Structural magnetic resonance imaging in Parkinson disease with freezing of gait: A systematic review of literature. J Neurosci Rural Pract 2023;14:399-405.
Abstract
Objective:
This review aims to the existing structural neuroimaging literature in Parkinson disease presenting with freezing of gait. The summary of this article provides an opportunity for a better understanding of the structural findings of freezing of gait in Parkinson disease based on MRI.
Methods:
This systematic review of literature follows the procedures as described by the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results:
Initial searches yielded 545 documents. After exclusions, 11 articles were included into our study. Current findings of structural MRI on freezing of gait in Parkinson disease are associated with structural damage between sensorimotor-related cortical grey matter structures and thalamus, but not cerebellum and smaller systems, as well as extensive injuries on white matter connecting between those structures.
Conclusion:
Current findings of structural MRI on freezing of gait in Parkinson disease are associated with structural damage between sensorimotor-related cortical grey matter structures and thalamus, but not cerebellum and smaller systems, as well as extensive injuries on white matter connecting between those structures.
Keywords
Structural magnetic resonance imaging
Parkinson
Freezing of gait
INTRODUCTION
Parkinson’s disease (PD) is a well-known syndrome of muscle rigidity, resting tremor, bradykinesia, and postural balance problem.[1] Freezing of gait (FoG) is a condition that affects patients with PD. It is characterized by an episodic inability to produce effective stepping, which is brought on by an absence or noticeably reduced forward movement of the feet despite the patient’s intention to walk.[2-4] FoG may develop during disease progression, which stage is called PD with FoG (PDFoG). The cases of PD with incomplete clinical findings may need to be critically distinguished with Parkinsonism due to secondary cause and other insults toward basal ganglia.[3,5-7]
Diagnosis of PD-FoG still poses challenge when faced with anatomical, de facto criteria.[1] This is mainly due to the fact classical pathognomonic PD finding of dopamine deprivation in the substantia nigra which is mainly supported by gross anatomy findings from autopsy.[8] However, since not all PD developed into PD-FoG, it is predicted that there must be peculiarity of neuroimaging present only in PD-FoG compared to PD-non Freezing of Gait (PD-nFOG).[5] Study on normal human gait reflects the complex collaboration between intracranial structures such as sensorimotor cortex, thalamus, basal ganglia, midbrain, pons, cerebellum, and respective white matter in each walking phase.[4,9] Hence, the dogma where combination of damage between these organs during PD progressivity may reflect as PD-FoG.
Current neuroimaging approach of PD is using magnetic resonance imaging (MRI).[5] MRI in PD has been a staple neuroimaging modality to assess basal ganglia and its correlating structures. Functional MRI (fMRI) has been developed to assess brain activities under resting condition or under specific load of task. The fMRI for PD is able to differentiate activities between subcomponent activation of basal ganglia, distinguishing activities between grey matter and white matter, and analyzing white matter activities between sensorimotor cortex, tracts, thalamus, and basal ganglia.[4] However, fMRI is proven to be hard to do for PD-FoG diagnosis since ideal PD-FoG fMRI requires leg movement to activate the brain parts involved; hence, the resting and post-walking fMRI technique which did not fully reflect the PD-FoG under walking attempt.[10] Moreover, in the case of health systems is limited to access fMRI, the cheaper structural MRI (sMRI) is often become the only option. The sMRI has been extensively used to evaluate abnormal brain structure related to PD;[10-12] however, correlation of sMRI result with PD-FoG based on current research findings is still yet to be understood.
MATERIALS AND METHODS
A systematic evaluation based on sMRI clinical trials on PDFOG was carried out. This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline. This study was registered with PROSPERO (CRD42023407920).
Eligibility criteria
To be included in this review, studies must meet some inclusion criteria. The studies must be analytical study which compares PD-FoG and PD-nFoG. Methods used must be MRI focusing on structural analysis with describable statistical analysis. The preferable outcome is significant and/or clinically important anatomical difference on any brain substructure. To expand our scope of findings, patients are not distinguished by whether they had received treatment or not. Studies are excluded if they are not a final publication, still on-review process, and not an analytical study. Duplicates are also excluded from this study. Derivatives from one research tree can be included if results are distinguishable compared to the main research.
Search strategy
Study was conducted using e-database of PubMed and ScienceDirect. We use keyphrase combinations of “Parkinson disease,” “Parkinson,” “sMRI,” “Magnetic Resonance Imaging,” “Structural Magnetic Resonance Imaging,” “Structural MRI,” and “Freezing of Gait.” The operator of “OR” and “AND” was used interchangeably between keyphrases.
Selection process
To fasten our findings as well as screen exclusion criteria earlier, built-in search engine filter for “randomized controlled trial” was used to automatically exclude reviews, literature reviews, case series, case report, and editorial letter. The filter of “Full Text” is applied to exclude abstract-only publication. Search yields then screened for duplicates or possible derivative studies. Authors then read the title abstract of the filtered study to screen for appropriate topic. The decision whether a study met the inclusion criteria was screened through each study methods section. The final decision whether a study is included then was carried by the senior author. For each applicable study, reference section is then screened for possible missed studies.
Data items and collection process
This study sought the data of sMRI comparison result in PD-FoG using any methods. Main data items sought were main author’s name, year of publication, study subjects, study design, study methods, and study’s important finding as described by main outcomes. Main outcomes are abnormalities on specific brain structures that were reported as described by sMRI findings on (i) location of abnormalities; (ii) statistically significant finding s by utilizing mathematical methods, and (iii) clinical significance based on reported conclusion. These were by done reading the entirety of manuscripts. Two reviewers collected data from each report, all worked independently.
Effect measures
If applicable, mean difference was the main point of mathematical effect measures for this study. It must be noted that mean difference may vary between studies since detected abnormality’s location between study subjects may vary. Significance is regarded as P < 0.05. Confidence interval of 95% was used if P-value is not used.
Synthesis methods
Obtained data for systematic review then tabulated into recapitulation table. Due to expected variability of anatomical findings, direct comparison may become difficult. Thus, each finding was subject to descriptive-only synthesis. Statistical analysis was not performed in this study.
RESULTS
Study selection
We obtained 545 studies through the aforementioned search strategy, which comprised as 509 studies through ScienceDirect database and 36 studies through PubMed database. Among those, 16 were selected because it fulfills inclusion criteria. The excluded studies were two studies which are abstract-only, six studies which are literature review, and one study because it is a research protocol-only. There were five studies on PD which includes FoG as subject of research, but did not group it into exclusive group. The remaining studies were excluded because the partially recognized key phrase (e.g., Parkinson Disease study but without FoG topic, or PD study but no relation with MRI topics). From the 16 studies, four studies were excluded due to duplicates. Finally, 11 studies were selected to be included in this review [Figure 1].
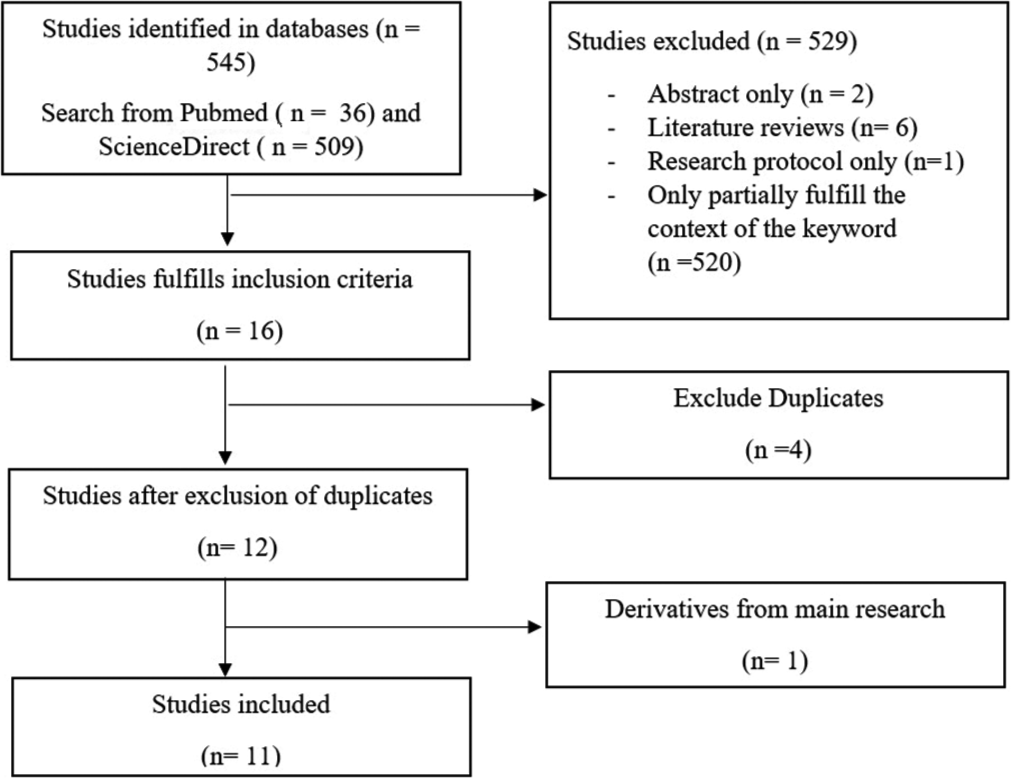
- Selection process preferred reporting items for systematic reviews and meta-analyses flow diagram.
Included and discussed studies are Snijders et al., Kostic et al., Tessitore et al., Sunwoo et al., Canu et al., Jha et al., Myers et al., Vastik et al., Pietracup et al., and Bharti et al. which are case–control study and D’Cruz et al. which is prospective cohort study [Table 1].
| S. No. | Authors | Year | Study design | Population | Methods | Important findings |
|---|---|---|---|---|---|---|
| 1. | Snijders et al.[14] |
2011 | Case-control study | Thirteen PD-FOG patients and 12 PD-nFOG patients | • Volume-based morphometry using statistical inference at cluster and voxel level, with anatomical inference using Statistical Parametric Map Anatomy Toolbox and WFU PickAtlas Tool version 2.4 | • Grey matter loss in the mesencephalic locomotor region in PD-FoG group • No differences in global grey matter, white matter or CSF volume between groups |
| 2. | Kostic et al.[15] |
2012 | Case-control study | Seventeen PD-FoG patients, 20 patients with PD-nFoG patients | • WMHs identified on dualecho scans and measured using Jim 4.0 (http://www.xinapse.com/Manual/index.html) • Voxel-based morphometry performed using SPM8 and the DARTEL registration method |
• More grey matter atrophy of bilateral dorsal frontal cortex, caudate nuclei, left midcingulate cortex, right hippocampus/amygdala, bilateral middle temporal gyrus, left inferior and right superior temporal gyri, bilateral inferior and superior parietal cortex, right precuneus, bilateral postcentral gyrus, left middle occipital cortex and cuneus, and right superior occipital gyrus in PD-FoG patient |
| 3. | Tessitore et al.[16] |
2012 | Case-control study | Twelve PD-FoG patients, 12 patients with PD-nFog | • Voxel-based morphometry using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm), and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) with default parameters incorporating the DARTEL toolbox in SPM8 | • PD-FoG showed a reduced grey matter volume in the left cuneus, precuneus, lingual gyrus, and posterior cingulate cortex compared with both patients with PD-nFoG • Correlation of PD-FoG clinical severity with GM loss in posterior cortical regions • No differences in global grey matter, white matter, or CSF volume between groups |
| 4. | Sunwoo et al. [17] |
2013 | Case-control study | Forty-six non-demented patients with PD, categorized into PD-FoG (n=16) and PD-nFoG (n=30) groups | • Manual delineating the boundaries of substantia innominata with MRIcro software on the coronal T1-weighted MRI scans • Volumetric analysis for subcortical structures was performed with FSL eFMRIB’s Integrated Registration and Segmentation Tool, version 5.0 |
• Normalized substantia innominata volume did not differ significantly between the PD-FoG and PD-non-FoG group • Thalamic volumes were significantly reduced in PD-FoG compared with PD-non-FoG after adjusting for age, sex, disease duration, the Unified PD Rating Scale scores and total intracranial volume |
| 5. | Canu et a.l[13] |
2015 | Case-control study | Twenty-three patients with PD-FoG compared with PD-nFoG | • Detection of grey matter volume alterations: VBM using SPM8 (www.fil.ion.ucl.ac.uk/spm/) and the DARTEL registration method • White matter damage analysis: DT MRI analysis: FSL tools (http://www.fmrib.ox.ac.uk/fsl/fdt/) and the JIM5 software (Version 6.0, Xinapse Systems, Northants, UK, http://www.xinapse.com). • Multisubject DT MRI analysis for tract-based analysis: TBSS version 1.2 (http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) |
• No grey matter atrophies • Bilateral white matter damage of the pedunculopontine tract, corpus callosum, corticospinal tract, cingulum, superior longitudinal fasciculus, and white matter underneath the primary motor, premotor, prefrontal, orbitofrontal, and inferior parietal cortices • Right pedunculopontine tract damage is associated with a greater disease severity. • White matter damage of the genu and body of the corpus callosum and right parietal |
| 6. | Jha et al.[18] | 2015 | Case-control study | Seventeen PD-FOG and 21 PD-FoG patients | • Vertex-based morphometry and Statistical Parametric Mapping 8 for grey matter analysis | • Significant grey matter atrophy in PD-nFoG group in left temporal, right frontal areas and significant involvement of right cerebellum. |
| 7. | Myers et al.[19] |
2017 | Case-control study | Sixty-three participants were divided into two groups, PD-FoG (n=25) and PD-nFoG (n=38) |
• Cortical and cerebellar lobular segmentation analysis with FreeSurfer software (v5.3.0, http://surfer.nmr.mgh.harvard.edu/) and the SUIT toolbox[3,19]) a complete cognitive dataset. | • No differences in cerebellar volumes between PD-FoG and PD-nFoG groups |
| 8. | Vastik et al.[20] |
2017 | Case-control study | Twenty-one patients with PD, 11 with FOG and 10 without FOG | • Surface-based morphometry implemented in FreeSurfer to quantify the grey matter atrophy. | • Significant cortical atrophy in PD-FoG’s left supplementary motor area (BA 6), middle/ anterior cingulate cortex, temporal pole (BA 38) and frontal operculum • Significant differences in the left mid-anterior cingulate cortex, • Significant cortical reduction in more posterior portions of the cingulate cortex bilaterally • The most affected structures were the anterior and midanterior cingulate, and superior frontal gyrus (SMA) |
| 9. | Pietracup et al.[21] | 2017 | Case-control study | Twenty-one PD-FoG patients, 16 PD-nFoG patients | Grey matter measurement using SBM using FreeSurfer pipeline White matter measurement using TRACULA toolbox |
• No significant differences in Cortical Thickness between PD-FoG and PD-nFoG • Significantly smaller surface area in the right parietal cortex, namely in the postcentral and supramarginal gyri and the superior parietal lobule in PD-FOG patients • No differences in subcortical volumes were found between PD-FOG and PD-nFOG patients. |
| 10. | Bharti et al.[22] |
2019 | Case-control study | Fifteen patients with PD-FOG versus 16 patients with PD-nFOG | • FMRIB’s diffusion toolbox (http://fsl.fmrib.ox.ac.uk) for automatic subcortical segmentation program • TBSS tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) for voxel-wise statistical analysis of fractional anisotropy mapping • SUIT version 3.2 implemented in SPM12 for cerebellar volumetry |
• Abnormality on cerebellar peduncle: the PD-FoG exhibited lower fractional values in the superior cerebellar peduncle and medial cerebellar peduncle than PD-nFOG • Higher mean diffusivity values in the superior cerebellar peduncle of PD-FoG compared to PD-nFoG • No global or lobular cerebellar volume differences between PD-FoG and PD-nFoG |
| 11. | D’Cruz et al.[23] |
2021 | Prospective cohort study | Fifty-seven individuals with PD-FoG compared with PD-nFoG and PD-nFoG to FoG convert | • Vertex-based analysis of grey matter morphology | • Local inflations in bilateral thalamus |
sMRI: Structural magnetic resonance imaging, WMHs: White matter hyperintensities, FSL: FMRIB software library, BA: Brodmann area, TBSS: Tract-based spatial statistical, SUIT: Spatially unbiased infratentorial toolbox, DT MRI: Diffusion tensor magnetic resonance imaging, SBM: Surface-based morphometry, TRACULA: Tracts constrained by underlying anatomy, PD-FoG: Parkinson’s disease freezing of gait, PD-nFoG: Parkinson’s disease new freezing of gait, DARTEL: Diffeomorphic anatomic registration exponentiated lie algebra, CSF: Cerebrospinal fluid
Study characteristics
The obtained study publication was spanned from year 2010 through 2022. All of the studies at least distinguish between FoG and non-FoG group, with healthy subjects occasionally presented as negative control group. Study designs varied greatly with main trend of using PD-FoG versus PD- nFoG versus “Negative Control Group” grouping style in which leads to case–control design. Study by D’Cruz et al. provides third group, “FoG converts” which provide information on people with PD who developed PD-FoG. Most subjects in the studies are divided into 1:1 group ratio, signaling the attempt to comply with case–control design. Usage of cohort study of sMRI in PD-FoG is rare (n = 1). MRI machine specification is described by Bharti et al., Tessitor et al., Vastik et al., and Kostic et al., which provides information of 1.5–3 T MRI as research instrument standard. Every study gives heavily detailed protocols on how to obtain statistically analyzable data, which are mainly grouped into two methods: (1) Voxel-based morphometry for grey matter analysis and (2) either TBSS or tractography for white matter analysis.
Risk of bias
All studies showed how sample was obtained and how PD was diagnosed, as well as criterion for determining FoG. However, nine studies did not report whether sMRI expert and statistical analyst was blinded during study. We did not find any indication of publication and reporting bias.
Methods for assessing the risk of bias using the strengthening the reporting of observational studies in epidemiology checklist for included studies
Individual studies results
Individual studies results are summarized on [Table 1].
Synthesis results
All studies, while differs greatly in study design, aimed to analyze difference of brain structure between PD-nFoG and PD-FoG group. Most of the studies (n = 9) provide result based on comprehensive sMRI findings rather than single, preferred target outcome. Results of analysis are divided mainly into two categories: (1) Grey matter volumetry, which includes vertex-based and surface-based volumetric analysis, and (2) white matter analysis which includes spatial analysis based on fractional anisotropy and mean diffusivity mapping. There is a contradictory study report where D’Cruz study[23] reported local inflations in bilateral thalamus of patients with PD-FoG,[22] while Sunwoo et al. reported significant reduction of thalamic volume. Based on result adjusted to at least patient age and sex, most volumetry studies agreed that there is no difference between grey matter volume caused by atrophies between PD-FoG and PD-nFoG group. Snijder et al. and Tessitore et al. revealed no differences between PD-FoG and PD-nFoG groups in white matter, global grey matter, or cerebrospinal fluid volume. The corpus callosum, cingulum, corticospinal tract, superior longitudinal fasciculus, and white matter beneath the primary motor, prefrontal, premotor, orbitofrontal, and inferior parietal cortices all displayed damage as a result of the combined effects of all white matter analyses.
DISCUSSION
FoG is a disabling phenomenon that frequently affects people with PD. The PD-FoG is thought to involves extensive part of the brain, including primary sensory cortex, primary motor cortex, basal ganglia, thalamus, midbrain, and cerebellum as well as white matter that connects these areas.[4,7,10] While therapy of PD is well-defined, it seemed that in PD-FoG these therapies are not as effective as in PD alone. Therefore, understanding the structural involvement of each brain structure in PD-FoG is critical to develop better treatment toward patient.
Patient gait and mobility is based on heavily coordinated, both voluntary and involuntary steps happening in the brain.[9] First, activated primary motor cortex provides input through pyramidal system for a side of leg to do hip flexion and contralateral hip extension, which contributes to first attempt of movement. This is followed by proprioceptive input that passes through the thalamus, stimulating prefrontal cortex to produce critical input for extensive and complex involuntary movements which includes swing of the knee, precisive heel-strike, and, finally, precisive alignment of the foot on floor topology. This is also followed by similar contralateral leg movement which produces walking movement. These smooth involuntary movements are thought to be controlled by either direct or indirect input toward basal ganglia, where extrapyramidal tract plays major role. The involvement of proprioception and balance suggests active role of cerebellum and pedunculopontine tracts. Synchronization between two legs suggests active coordination between contralateral hemispheric cortexes, which suggest the role of corpus callosum. Hence, in the case of PD-FoG, it is expected that components of this system are affected under certain degree of damage.
Volumetric sMRI study on grey matter is and extensive research subject for PD-FoG, as well as the PD itself. At present, the target of volumetric sMRI study in PD-FoG is primary motor and sensory cortex, globus pallidus, striatum, thalamus, and subthalamic nuclei.[4,6,13] Volumetric sMRI studies suggest that brain atrophy is the main culprit of FoG development in PD. However, it is still unclear whether brain atrophy is a part of PD progression, or the part of neurodegeneration as a whole.[5] Damage in the grey matter of basal ganglia was initially thought to be the main cause of FoG. However, it should be noted that isolated grey matter damage of basal ganglia components as well as subthalamic nuclei may produce hyperkinetic symptoms instead, such as ballismus, chorea, and athetosis. This recalls the function of extrapyramidal system itself, where it relays and smoothen main motor input initiated through primary motor cortex. Hence, from the sMRI of PD-FoG, it is expected to reveal normal to minimal damage on grey matter of extrapyramidal system and subthalamic nuclei, but may reveal extensive atrophies on primary motor cortex grey matter. Recent studies findings on sMRI alone also showed that compared to PD-nFoG, there is no difference on cerebellar grey matter volume in PD-FoG, suggesting preservation of cerebellar control but the existence of cerebellar white matter-related insult based on structure and connectivity of cerebral cortex. This also suggests that in PD-FoG, pathology might lie in white matter connectivity while grey matter structure itself is preserved.
Study on white matter seemed to help reveals the FoG pathology more compared to the grey matter volumetry. White matter is the tract-based region of the brain. It connects between grey matter areas and serves as hotspots for input transmission. Damage of white matter is quite disabling in a long term, since reduction from input feedback is known to lead toward grey matter atrophy. Tract-based sMRI analysis suggest that in PD-nFoG, progressive damage of tract connecting frontal and basal ganglia plays role in the progressivity and onset of FoG.
Concern is raised on sMRI research result when it comes to the methods. Unlike therapeutic studies, diagnostic studies on MRI are subject to study design, limited participants, rarity of disease, disease progression, machine specification, and diversity of statistical methods which often make a review and meta-analysis difficult. Even though if we overcame these obstacles, the current dogma of reporting for radiological result is to “spot the obvious, read as a whole” often ends up in highly varied outcomes on a single MRI study. This may give advantages in seeing PD as a whole disease, but may slow the focus on specific findings and objectives.
CONCLUSION
The finding of this review allows us to conclude that sMRI is a useful tool to assess PD-FoG. New findings on sMRI of PDFoG provide information that FoG in PD is white matter-heavy pathology rather than the effect on previously thought grey matter-dominant pathology. Understanding on how the white matter damage is developed in PD-FoG and how it is prevented is the further objective of research on PD, as well as potential target for future therapies.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Challenges in the diagnosis of Parkinson 's disease. Lancet Neurol. 2021;20:385-97.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait in Parkinson's disease: Where are we now? Curr Neurol Neurosci Rep. 2013;13:350.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait in Parkinson's disease reflects a sudden derangement of locomotor network dynamics. Brain. 2019;142:2037-50.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait in Parkinson's disease: Pathophysiology, risk factors and treatments. Transl Neurodegener. 2020;9:12.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging advances in Parkinson's disease and atypical Parkinsonian syndromes. Front Neurol. 2020;11:572976.
- [CrossRef] [PubMed] [Google Scholar]
- Gait and postural disorders in Parkinsonism: A clinical approach. J Neurol. 2020;267:3169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait: Understanding the complexity of an enigmatic phenomenon. Brain. 2020;143:14-30.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46:S30-3.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging of freezing of gait. J Parkinsons Dis. 2015;5:241-54.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging advances in Parkinson's disease with freezing of gait: A systematic review. Neuroimage Clin. 2019;24:102059.
- [CrossRef] [PubMed] [Google Scholar]
- Brain MRI in Parkinson's disease. Front Biosci (Elite Ed). 2014;6:360-9.
- [CrossRef] [PubMed] [Google Scholar]
- Brain structural and functional connectivity in Parkinson's disease with freezing of gait. Hum Brain Mapp. 2015;36:5064-78.
- [CrossRef] [PubMed] [Google Scholar]
- Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. 2011;134:59-72.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409-16.
- [CrossRef] [PubMed] [Google Scholar]
- Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol. 2012;33:1804-9.
- [CrossRef] [PubMed] [Google Scholar]
- Thalamic volume and related visual recognition are associated with freezing of gait in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord. 2013;9:1106-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychological and imaging profile of patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord. 2015;21:1184-90.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebellar volume and executive function in Parkinson disease with and without freezing of gait. J Parkinsons Dis. 2017;7:149-57.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait is associated with cortical thinning in mesial frontal cortex. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:389-96.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of gait in Parkinson's disease: Gray and white matter abnormalities. J Neurol. 2018;265:52-62.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal cerebellar connectivity patterns in patients with Parkinson's disease and freezing of gait 2019. Cerebellum. 2019;18:298-308.
- [CrossRef] [PubMed] [Google Scholar]
- Thalamic morphology predicts the onset of freezing of gait in Parkinson's disease. NPJ Parkinsons Dis. 2021;7:20.
- [CrossRef] [PubMed] [Google Scholar]







