Translate this page into:
Intramuscular ketamine provides better sedation and scan conditions in children undergoing magnetic resonance imaging: A single-blinded observational study
*Corresponding author: Pooja Thaware,Department of Anaesthesiology and Critical Care, L N Medical College and J K Hospital, Bhopal, Madhya Pradesh, India. pthaware20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jain A, Kaushal A, Trivedi S, Thaware P, Chaudhary N, Jain S. Intramuscular ketamine provides better sedation and scan conditions in children undergoing magnetic resonance imaging: A single-blinded observational study. J Neurosci Rural Pract 2023;14:477-81.
Abstract
Objectives:
The objectives of this study were to compare the quality of sedation provided by intravenous (i.v.) and intramuscular (im) ketamine for pediatric magnetic resonance imaging (MRI).
Materials and Methods:
This study was a non-randomized, single-blinded, and prospective observational study. After receiving approval from the Institutional Ethics Committee, a total of 108 children aged 2–7 years were divided into two groups, with 54 children in each group. In the i.v. group, children received ketamine at a dose of 1.5 mg/kg intravenously, while in the im group, children received ketamine at a dose of 4 mg/kg intramuscularly. If a Ramsay sedation score of 6 (RSS-6) was not achieved, half of the loading dose of ketamine was repeated. In both groups, rescue propofol boluses of 1 mg/kg intravenously were administered whenever the child moved. The primary outcome measure was the quality of sedation, which was assessed by a blinded radiologist. The time taken to reach RSS-6, the number of rescue propofol boluses, the total time wasted in taking repeat sequences, and the time required to achieve a modified Aldrete score of 9 (MAS-9) were recorded.
Results:
The im group demonstrated significantly better sedation quality. In the i.v. group, the time to achieve RSS-6 was significantly shorter, but it required more rescue propofol boluses to maintain sedation. The i.v. group also experienced a notable increase in the total time wasted during repeat sequences. On the other hand, the i.v. group exhibited a shorter time to reach MAS-9 compared to the im group.
Conclusion:
The im group showed superior sedation quality when compared to the i.v. group. However, it is important to consider that the im group experienced a longer recovery time.
Keywords
Anesthesia
Child
Ketamine
Magnetic resonance imaging
Outpatient anesthesia
INTRODUCTION
Ketamine is a commonly utilized medication for procedural sedation and the method of parenteral administration is determined by the preference of the anesthesiologist and the available research on pediatric procedural sedation.[1] The pharmacokinetics of ketamine vary depending on the mode of administration. The intravenous (i.v.) route of administration results in a quicker onset; however, the duration of action is shorter. On the other hand, intramuscular (im) administration necessitates a higher dose and produces a more prolonged sedation effect. There is limited research available that compares the im and i.v. routes of ketamine administration for pediatric sedation during magnetic resonance imaging (MRI). The main objective of the study is to assess and compare the sedation quality, as determined by a blinded radiologist, in relation to the imaging quality achieved.
MATERIALS AND METHODS
The study utilized a prospective non-randomized observational design. The study was conducted at a medical institute in central India. The study received approval from the Institutional Ethics Committee and was registered with the Clinical Trial Registry of India. The study took place between April 2021 and September 2021. All procedures adhered to the principles outlined in the Helsinki Declaration of 1975, as revised in 2000.
This study included patients between the ages of 2 and 7, of any gender, who were classified as American Society of Anesthesiologists physical status I or II, and were scheduled to undergo an MRI. Participation in the study necessitated written informed consent from the guardian.
Patients who were excluded from this study comprised those whose guardians declined to provide written informed consent, as well as children with a history of allergic reactions to any of the drugs used in the study. Moreover, patients classified as American Society of Anesthesiologists physical status III and IV, including those with raised intracranial pressure, were also excluded. In addition, individuals with anticipated difficult airway or a psychiatric illness such as schizophrenia were not included in the study.
The main objective of the study was the quality of sedation, which was evaluated by a blinded radiologist based on the image quality. The secondary objectives in this study were the time required to attain a Ramsay sedation score 6 (RSS-6), the number of rescue propofol boluses (1 mg/kg i.v.) needed to maintain adequate sedation, the time lost due to taking repeat sequences, and the time required to reach a modified Aldrete score (MAS) of 9.
The sample size of 108 participants (54 in each group) was determined based on the previous literature related to the mean distress score as the outcome variable. The statistical power was set at 80%, with a type I error level (α) of 5% and a type II error rate (β) of 20%.[2] In this study, statistical significance was defined as having P-value below 0.05.
After obtaining informed consent from the guardian, patients were enrolled in the study. The allocation of patients into either the i.v. group or the im group was determined at the discretion of the supervising anesthesia faculty.
The usual pre-anesthetic protocol was followed in all the patients, which included 6-h fasting period for solid food and a 2-h fasting period for clear liquids. Patients on antiepileptic medications were instructed to continue taking these medications on the day of the procedure. Baseline measurements for heart rate, blood pressure, respiratory rate, and oxygen saturation were recorded in the pre-procedure area. In all patients, a 22/24G venous cannula was secured.
The time required for the MRI examination of every patient was noted; this time started and ended with the placement and removal of the patient from the MRI table. Hemodynamic and respiratory monitoring was done throughout the MRI scan.
Thirty minutes before the procedure, ondansetron 0.1 mg/kg i.v. was given in both groups. Glycopyrrolate 5 µg/kg and midazolam 0.05 mg/kg i.v. were given in both the groups 15 min and 3 min, respectively, before administering ketamine. Patients were shifted to the MRI suite. In the MRI suite, the children in the i.v. group received ketamine 1.5 mg/kg i.v. and the children in the im group received ketamine 4 mg/kg im in the anterolateral area of the thigh.
The level of sedation was evaluated using the Ramsay sedation scale.[3] The goal was to achieve an RSS-6. RSS was assessed at 30 s intervals. After loading dose of ketamine, if RSS-6 was not achieved within 3 min and 10 min in the i.v. and im groups, respectively, half of the loading dose was repeated. The repeat dose of ketamine is administered through the same route as the loading dose. The time taken to achieve RSS-6 was noted. After achieving RSS-6, the patient is positioned for the MRI scan, and oxygen is given by face mask at 5 L/min. The positioning of the patient barely wasted any time.
If the patient moved amidst the procedure, rescue doses of propofol 1 mg/kg i.v. were given in both groups. The duration required to complete the scan was recorded. The number of repeated sequences and the time taken to complete them were noted.
A radiologist who was blinded to the group allocation rated the quality of sedation based on image quality; “poor” meant blurred image in 4 or more sequences, “good” meant blurred image in 3 or fewer sequences, and “excellent” meant no blurring.
After the procedure, the patients were shifted to the recovery room. The patient’s recovery was evaluated utilizing a MAS. The time taken to achieve MAS-9 or more after the end of the procedure was noted. MAS ≥ 9 represents a patient who was fully awake, able to cough freely and breathe deeply and maintaining oxygen saturation above 94% on room air, has a blood pressure within 20% of the baseline values, and can move all 4 or 2 extremities voluntarily or on command.
The adverse events during and after the procedure were recorded and treated. Bradycardia is defined as a decrease in heart rate of <60 beats/min. Bradycardia was treated with i.v. atropine 0.01 mg/kg. Pediatric Advanced Life Support guidelines define hypotension in 1–10 year aged children as a systolic blood pressure value <2 times the age added to 70 mmHg.[4] Hypotension was treated with ringer lactate 10 mL/kg i.v. bolus. Hypotension non-responsive to the fluid bolus is treated with mephentermine (3 mg i.v.).
Desaturation (oxygen saturation <94%), laryngospasm, and apnea (cessation of respiration for 20 s) were noted and treated by lifting the jaw, insertion of the nasopharyngeal or oropharyngeal airway, positive pressure ventilation, or intubation. Other adverse events such as vomiting, which were treated with i.v. ondansetron, excessive salivation requiring suctioning, emergence of delirium, involuntary movements, and convulsions were noted and treated appropriately.
Statistical analysis
Statistical analysis was conducted using version 20.0 of the Statistical Package for the Social Sciences software program. Continuous variables were analyzed using a two-tailed student’s t-test. Non-parametric data were assessed using Wilcoxon U-test. Ordinal data were analyzed using the Chi-square test or Fischer exact test wherever applicable. P < 0.05 was considered significant.
RESULTS
The patient enrolment flow diagram is shown in [Figure 1]. In each group, 35 patients had an MRI brain for developmental delay, seizure disorder, acute onset weakness, or infectious etiology; 15 patients had an MRI spine for spinal cord or vertebral column abnormality or infectious etiology; and four patients had an MRI limb for mass, limb deformity, or fracture.
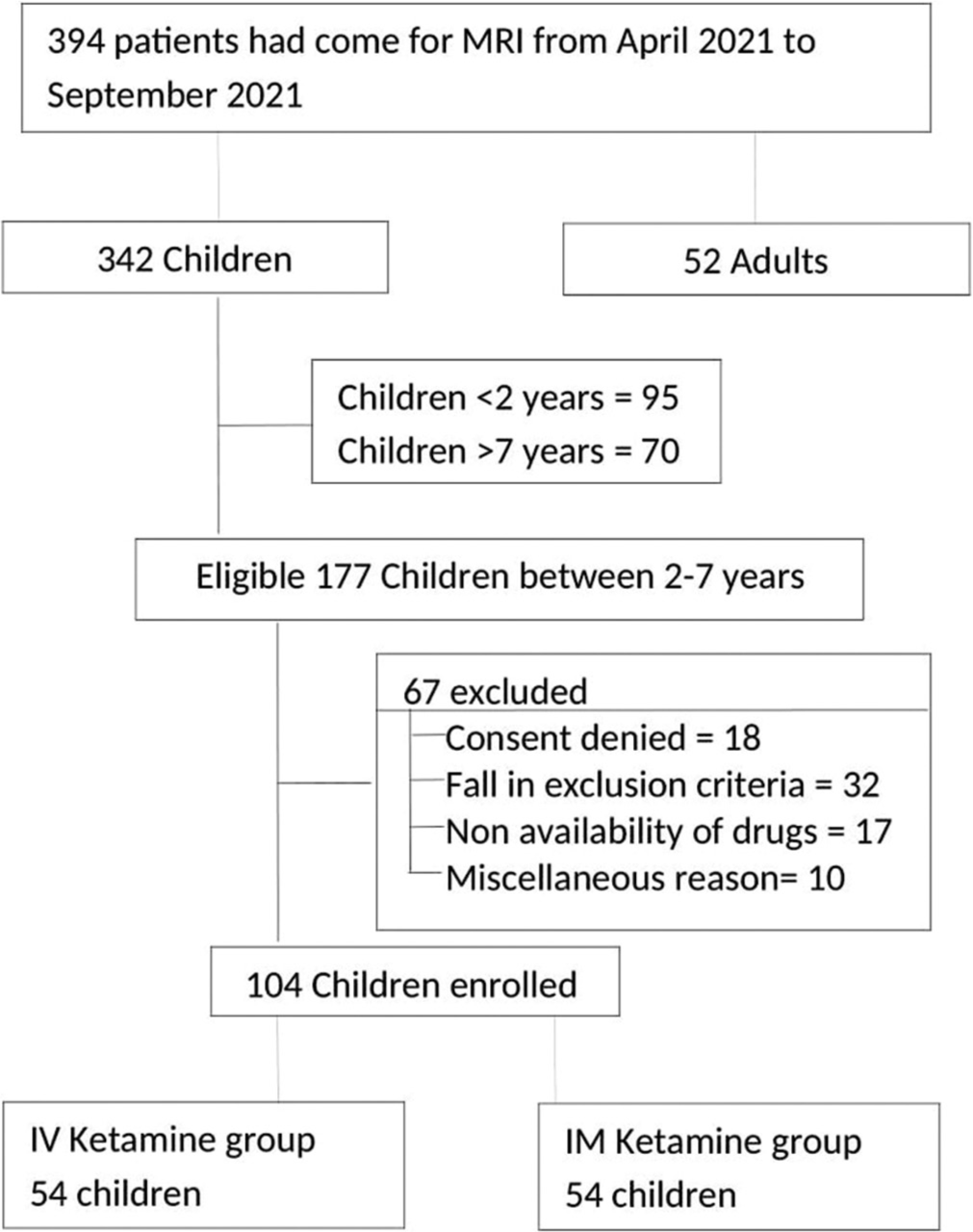
- Flow diagram depicting the patient enrolment for the study.
The two groups were demographically comparable for age, weight, gender, and duration of MRI [Table 1]. The quality of sedation as adjudged by a blinded radiologist was significantly better in the im group in comparison to i.v. group; the number of patients not showing blurring in any sequences was significantly higher in im group (34 vs. 18, P = 0.002).
| Intravenous group (n=54) | Intramuscular group (n=54) | P-value | ||
|---|---|---|---|---|
| Age in years* | 3.9±2.0 | 4.7±1.7 | 0.421 | |
| Male: Female | 36:18 | 40:14 | 0.412 | |
| Weight in kg* | 14.6±4.7 | 17.2±5.1 | 0.143 | |
| Mean duration of MRI in minute* | 44.1±24.1 | 43.7±19.5 | 0.877 | |
| Time to Achieve RSS-6 in min§ | 2.1±1.8 | 8.46±2.0 | 0.002 | |
| Number of patients requiring additional bolus at induction | 27 | 17 | 0.031 | |
| Median number of rescue propofol bolus (Interquartile range)11 | 2.5 (2-4) | 2.0 (2-4) | 0.491 | |
| Number of patients requiring repeat sequence | 28 (20 patient needed 1 sequence; 8 needed more than 1 sequence) | 11 (10 patient needed 1 sequence; 1 needed more than 1 sequence) | 0.0006 | |
| Total time wasted in taking repeat sequences | 148 min | 52 min | 0.013 | |
| Sedation quality as judged by the radiographer | Good=36, Excellent=18 | Good=20, Excellent=34 | 0.002 | |
| Mean time to achieve modified Aldrete acore 9 in min1 | 2.58±3.0 >10 min=10 patients 5-1 0 min=17 patients <5 min=27 patients |
7.8±6.0 >10 min=15 patients 5-1 0 min=30 patients <5 min=9 patients |
0.008 | |
The time taken to achieve an RSS-6 was significantly shorter in the i.v. group (P = 0.002). However, at the used doses, in the i.v. group, a significantly greater number of patients needed a repeat dose of ketamine over and above the initial induction dose (27 vs.17; P = 0.031) to achieve RSS-6.
At the used doses, the i.v. group needed more rescue boluses of propofol to maintain the sedation during MRI; the difference was not significant (P = 0.491).
Due to the blurring of the image, repeat MRI sequences had to be taken in 28 patients in the i.v. group and in 11 patients in the im group (P = 0.0006). Total time wasted in taking the repeat sequences was significantly more in the i.v. group (P = 0.013).
The mean time to achieve a MAS-9 was significantly longer in the im group (P = 0.008).
None of the patients in either group revealed bradycardia, hypotension, desaturation, laryngospasm, apnea, vomiting, excessive salivation, or any other adverse event.
DISCUSSION
This study at a single medical center compared the use of im and i.v. ketamine for sedating children during MRI scans. The findings of this study demonstrate that im ketamine at 4 mg/kg provides a superior quality of sedation and in turn better image quality than i.v. ketamine at 1.5 mg/kg. The superior sedation quality resulted in need of fewer rescue propofol boluses for the maintaining adequate sedation. An adequately powered study can further elucidate this difference in the propofol boluses.
When using im ketamine, it took longer to reach the desired level of sedation where there was no response to light glabellar tap or loud auditory stimulus (RSS-6), the excess time taken was approximately 6.3 min. The difference was due to the different pharmacokinetic properties of the drug when administered by different routes.[5]
Several studies support the use of ketamine in procedural sedation.[6-10] Ketamine in a dose as small as 0.5 mg/kg i.v. has been found to reduce the induction and maintenance doses of propofol and thus reduce the chance of sedation-related minor and major side effects. Ketamine can effectively reduce the occurrence of involuntary movement linked to propofol administration.[11] The ketamine benzodiazepine combination provides comparable scan conditions and is much less costly than a combination of benzodiazepine and fentanyl.[12]
Parenteral administration of ketamine by the im route has been studied mostly in emergency department setup and frequently for suturing, foreign body removal, and closed reduction of fractures. Many investigators have compared i.v. and im ketamine for procedural sedation in emergency department, but the dose of the main drug as well as the adjuvant has been quite varied, making the comparison of results difficult.
A randomized controlled trial conducted by Roback et al. compared i.v. (1 mg/kg) and im (4 mg/kg) ketamine in children for brief orthopedic procedures.[2] They reported a higher incidence of respiratory adverse events in the i.v. group (8.3% vs. 4.0%) and a higher incidence of vomiting in the im group (26.2% vs. 11.9%). In our study, we did not encounter any adverse events. In their study, the length of sedation was also longer in the im group (129 vs. 80 min). The median duration of the procedure was 13 min; even for such short-duration procedures, a greater number of patients in the i.v. group needed administration of multiple doses of i.v. ketamine in comparison to im group in their study (27% vs. 9%).
In our study, the time taken to achieve MAS-9 in the im group was significantly longer than in the i.v. group, it seems to be of less practical importance in view of authors. In a retrospective study, Ramaswamy et al. in an emergency setup found that patients who received im ketamine (3–4 mg/kg) had a longer time to discharge than those receiving i.v. ketamine (1.5 mg/kg) (approximately 20 min longer).[13] However, since the study was retrospective in nature, the dose of ketamine and adjuvant could not be standardized. They noticed that excessive salivation was another problem (11%) in the im ketamine group, as against 1.7% in the i.v. group; this could have been due to the exclusion of anti-sialagogues from their routine premedication.
Gharavifard et al. compared 4 mg/kg im and 1.5 mg/kg i.v. ketamine for procedural sedation and analgesia in children for short and painful procedures in emergency department.[14] They found that the duration of adequate anesthesia with i.v. ketamine to be significantly shorter than that of im ketamine (20.6 ± 12.0 and 37.2 ± 11.8 min, P < 0.001). The time for discharge readiness of the patient in both the groups was comparable (65.3 ± 36.9 vs. 72.2 ± 14.5 min in i.v and im groups, respectively, P = 0.401). The requirement for rescue doses was notably higher in the i.v. group (26.7% vs. 10.0%, P < 0.001). They also found that the quality of anesthesia was comparable in both the groups; excellent anesthesia in 66% in i.v group and 70% im group. In our study, the need for rescue boluses of propofol was considerably higher than that mentioned by Gharavifard et al. (51% vs. 26.7% in the i.v. group and 20% vs. 10% in the im group).[15]
Green and Krauss considered that the observations of Roback et al. were merely due to a difference in the dose of ketamine used.[2,15] They believed that 1 mg/kg ketamine was a suboptimal dose when compared to a 4 mg/kg im dose, and thus, the results were incorrect. In our study, we used the usual recommended dose of i.v. ketamine (1.5 mg/kg) and hence removed that confounding factor as well.[16,17]
The administration of other sedatives along with ketamine also affects the dose requirement and makes the comparison of the results of the available studies difficult.[18]
The limitation of this study is its single-center design, which resulted in a limited sample size. To address this limitation, future research could consider conducting a large multicenter randomized double-blinded controlled trial.
CONCLUSION
In light of the study’s findings, it can be inferred that the sedation quality was found to be significantly better when ketamine was administered intramuscularly (4 mg/kg) than intravenously (1.5 mg/kg) for pediatric MRI. In MRI, the i.v. route is preferred due to the busy setup, but considering better image quality, im ketamine administration is a rationale alternative. Recovery time can be longer with im ketamine administration, authors believe that the difference is not of much practical/clinical importance.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Pediatric procedural sedation and analgesia in the emergency department: Surveying the current European practice. Eur J Pediatr. 2021;180:1799-813.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, controlled trial of i.v. versus i.m. ketamine for sedation of pediatric patients receiving emergency department orthopedic procedures. Ann Emerg Med. 2006;48:605-12.
- [CrossRef] [PubMed] [Google Scholar]
- Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656-9.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of clinical paediatric guidelines for hypotension with population-based lower centiles: A systematic review. Crit Care. 2019;23:380.
- [CrossRef] [PubMed] [Google Scholar]
- Onset and effect duration of intrabuccal space and intramuscular ketamine in pediatrics. Adv Biomed Res. 2018;7:91.
- [CrossRef] [PubMed] [Google Scholar]
- Anesthesia for magnetic resonance imaging in children: A survey of Canadian pediatric centres. Can J Anaesth. 2003;50:425.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial comparing sevoflurane and propofol in children undergoing MRI scans. Paediatr Anaesth. 2009;19:672-81.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of ketamine and propofol versus either agent alone for procedural sedation in the emergency department. Am J Health Syst Pharm. 2011;68:2248-56.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial evaluating low doses of propofol infusion after intravenous ketamine for ambulatory pediatric magnetic resonance imaging. Saudi J Anaesth. 2014;8:510-6.
- [CrossRef] [PubMed] [Google Scholar]
- Paediatric procedural sedation-a review and an update. Indian J Anaesth. 2007;51:169-75.
- [Google Scholar]
- Pretreatment with intravenous ketamine reduces propofol injection pain. Paediatr Anaesth. 2003;13:764-8.
- [CrossRef] [PubMed] [Google Scholar]
- Procedural sedation in children for magnetic resonance imaging--comparison between ketamine diazepam combination with midazolam fentanyl combination. Mymensingh Med J. 2010;19:60-5.
- [Google Scholar]
- Pediatric procedural sedation with ketamine: Time to discharge after intramuscular versus intravenous administration. Acad Emerg Med. 2009;16:101-7.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized clinical trial of intravenous and intramuscular ketamine for pediatric procedural sedation and analgesia. Emerg (Tehran). 2015;3:59-63.
- [Google Scholar]
- Should I give ketamine i.v. or i.m.? Ann Emerg Med. 2006;48:613-4.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449-61.
- [CrossRef] [PubMed] [Google Scholar]
- Premedication and induction of anaesthesia in paediatric patients. Indian J Anaesth. 2019;63:713-20.
- [CrossRef] [PubMed] [Google Scholar]
- Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:229-38.
- [CrossRef] [PubMed] [Google Scholar]







