Translate this page into:
Use of Preoperative Ependymal Enhancement on Magnetic Resonance Imaging Brain as a Marker of Grade of Glioma
Address for correspondence: Dr. Sumit Sharma, F-12, Resident Doctors Hostel, Sawai Man Singh Medical College, Jaipur, Rajasthan, India. E-mail: sumsha1984@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Neural stem cells within the subventricular zone (SVZ) are thought to be responsible for the origin and the heterogeneous nature of the gliomas. The relationship of the gliomas to the SVZ can be appreciated as ependymal enhancement on contrast magnetic resonance imaging (MRI). This study evaluates the rate of ependymal enhancement and its association with the histopathological grade of gliomas.
Patients and Methods:
Seventy-five patients with radiological features of glioma were recruited. Preoperative MRI was evaluated for the presence of ependymal enhancement and fluid-attenuated inversion recovery (FLAIR) signal proximity of tumor to ependyma, and the association to grade was investigated.
Results:
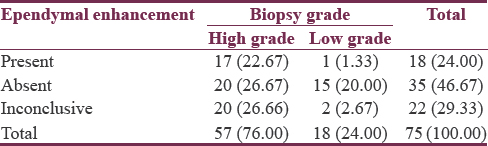
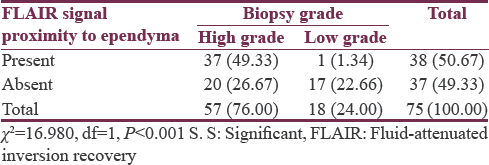
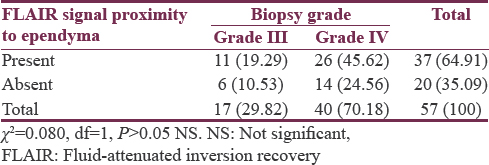
Seventy-five patients studied showed a male predominance (62.66%) with a mean age of 44.91 ± 13.64 years. Evidence of ependymal enhancement was positive in 24% (n = 18), 46.67% (n = 35) showed no evidence, and in 29.33% (n = 22), assessment was inconclusive. According to WHO grading, 76% (n = 57) were high-grade gliomas (HGGs) including Grade III (n = 17) and Grade IV (n = 40) and 24% (n = 18) were low-grade gliomas (LGGs) Grade II. HGGs were significantly associated with ependymal enhancement (P < 0.01) and FLAIR signal proximity to the ependyma (P < 0.001). Among HGGs, rate of ependymal enhancement and FLAIR signal proximity was more in Grade IV than Grade III but was not statistically significant (P > 0.05).
Conclusion:
SVZ is associated with HGGs. These MRI features can be helpful in predicting the tumor grade preoperatively and by including LGGs, the role of SVZ in the heterogeneous disease process of glioma can be studied as a whole, not only in the glioblastoma (GBM).
Keywords
Ependymal enhancement
fluid-attenuated inversion recovery
glioma
grading
magnetic resonance imaging
neural stem cells
subventricular zone
INTRODUCTION
The adult human brain harbors astrocyte-like neural stem cells (NSC) within the subventricular zone (SVZ), which is located just under the ependyma of the lateral ventricles and subgranular zone of dentate gyrus.[123] In animal models, gliomas – including glioblastoma (GBM) – can be induced from SVZ cells.[1] These data thus support the notion NSCs proliferate in the SVZ and are responsible for origin of gliomas.
Gliomas are heterogeneous, highly invasive primary brain lesions, and the grade of tumor plays a key role in determining treatment strategies. The primary treatment of low-grade gliomas (LGGs) is surgical excision. High-grade gliomas (HGGs) include Grade III and Grade IV tumors and require multimodality treatment. Response of HGGs to the treatment, especially of GBM, is highly variable. Heterogeneity may be related to the cells of origin in SVZ.[4] Magnetic resonance imaging (MRI) is commonly utilized as a part of the diagnostic workup for the clinical diagnosis and treatment planning of gliomas.[567] The current study was designed to test the rate of ependymal enhancement and fluid-attenuated inversion recovery (FLAIR) signal proximity of tumor to ventricular ependymal in preoperative MRI as a marker of grade and behavior of glioma to assess the relationship of the glioma with SVZ.
PATIENTS AND METHODS
Seventy-five patients with radiological features suggestive of supratentorial glioma, who were treated at our institution from December 2015 to December 2016, were included in the study. Ethical clearance from the Institutional Ethical Committee was obtained. Patients were included in the study if their contrast-enhanced MRI was suggestive of gliomas. Patients with posterior fossa lesions, any systemic malignancy, inadequate imaging, and histopathology other than gliomas were excluded from the study. Data recorded included the age of the patient, date of diagnosis, Karnofsky performance score (KPS) at the time of first visit, comorbidities, symptoms, any drug or contrast allergy, and MRI findings. Tumor grade was histologically confirmed in all cases in the Department of Pathology at our institution.
Most of the patients underwent the same preoperative MRI protocol, which consists of T1 fast field echo (FFE) (222/4.6 [TR/TE]), an axial sagittal and coronal T2W Turbo spin echo (2500/80 [TR/TE]), axial FLAIR sequence (10000/125/2800 [TR/TE/TI]), axial diffusion-weighted imaging (TR/TEd [2540/91]), and a postcontrast FFE T1. All MRI studies were reviewed by an experienced radiologist blinded to patient treatment protocols and histopathology. Tumors were classified as contrast-enhancing lesion bordering the ependyma and contrast-enhancing lesion not bordering the ependyma, and FLAIR signal proximity to the ependymal surface present or absent.
Based on ependymal enhancement on contrast MRI, cases were divided as follows - with evidence of ependymal enhancement present, ependymal enhancement absent, assessment of ependymal enhancement inconclusive due to mass effect, and ventricular compression that precluded accurate assessment. The last group was not used in calculations of ependymal enhancement. Based on FLAIR signal proximity to ependyma, cases were divided into two groups whether it was present or absent.
Histopathological grades of all the patients were examined, and grade of gliomas was seen with respect to the presence or absence of ependymal enhancement and with respect to FLAIR signal proximity to ependymal surface.
Statistical analysis
Data were analyzed using statistical software package SPSS Inc. Released 2007 and SPSS for Windows, version 16.0, SPSS Inc., Chicago, IL, USA. The qualitative data were analyzed using Chi-square test. The quantitative data were analyzed by Z-test. P < 0.05 was considered statistically significant.
RESULTS
In the present study, 75 cases of supratentorial gliomas with ages ranging from 12 to 75 years (mean age of 44.91 ± 13.64 years) were studied. Males were affected more (n = 47) than females (n = 28). Headache was the most common symptom, present in 69.33% (n = 52) cases followed by weakness 34.6% (n = 26). Seizures were present in 28% (n = 21) with generalized tonic–clonic in 25.33% (n = 19) and partial seizure in 2.67% (n = 2). Vomiting was present in 25.33% (n = 19). Eight percent (n = 6) of patients had difficulty in speech with 6.67% (n = 5) having motor aphasia and 1.33% (n = 1) with slurred speech, consciousness was altered in 13% (n = 10), and cranial nerve involvement was present in 4% (n = 3).
Karnofsky Performance score (KPS) was ≥70 in 70.67% (n = 53) patients and <70 in 22 29.33% (n = 22). Hypertension was the most common comorbidity, present in 18.67% (n = 14), followed by diabetes in 10.67% (n = 8) and chest infection in 1.33% (n = 1).
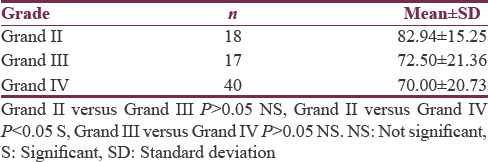
According to WHO grading, 18 (24%) cases were of Grade II, 17 (22.67%) of Grade III, and 40 (53.33%) were GBM-Grade IV. Grade III and Grade IV were high grade and Grade I and Grade II were low grade. There was no Grade I case in the study. Mean ± standard deviation (SD) age was 39.39 ± 12.07 years for Grade II (n = 18), 40.18 ± 13.41 years for Grade III (n = 18), and 49.40 ± 12.79 years for Grade IV (n = 40) gliomas [Table 1]. Mean KPS was 82.94 ± 15.25 for Grade II (n = 18), 82.94 ± 15.25 for Grade III (n = 17), and 70.00 ± 20.73 for Grade IV (n = 40) gliomas [Table 2].

Evidence of ependymal enhancement on MRI was present in 24% (n = 18) [Figures 1 and 2]. Enhancement was not present in 46.67% (n = 35) [Figure 3]. In 29.33% (n = 22), assessment was inconclusive [Figure 4 and Tables 3-5].
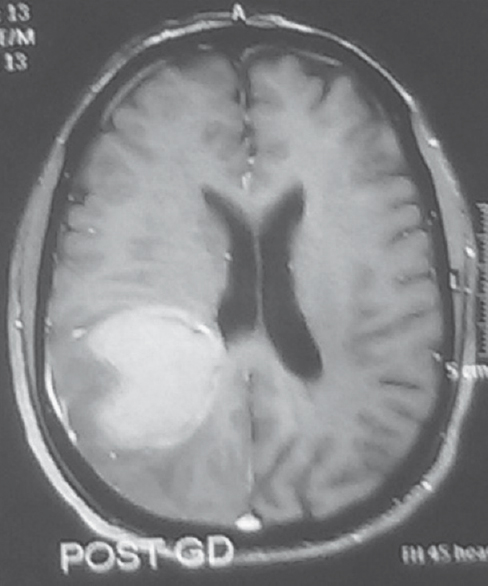
- Evidence of ependymal enhancement present. Axial gadolinium-enhanced T1-weighted image showing an enhancing tumor extending along the ventricular wall
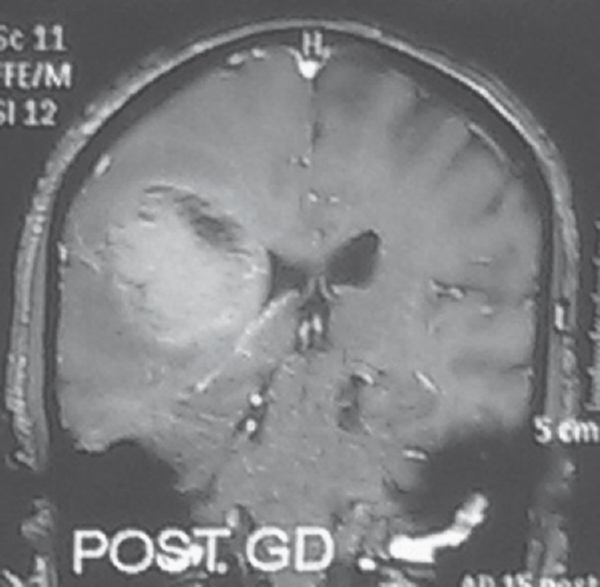
- Evidence of ependymal enhancement present. Coronal gadolinium-enhanced T1-weighted image showing an enhancing tumor extending along the ventricular wall
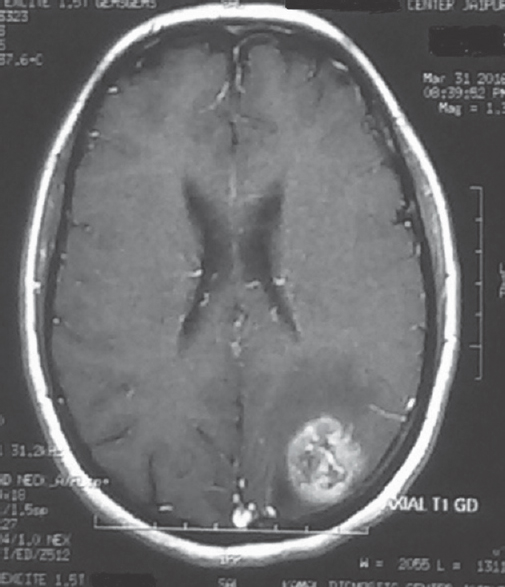
- No evidence of ependymal enhancement on magnetic resonance imaging axial gadolinium-enhanced T1-weighted image showing a left parietal lesion not contacting the ventricular wall
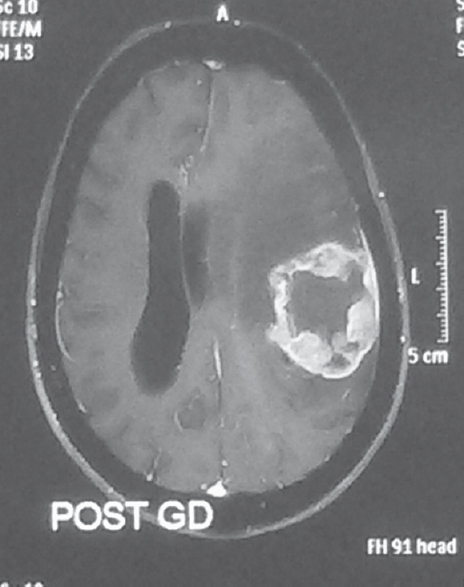
- Inconclusive for ependymal enhancement on magnetic resonance imaging. Axial gadolinium-enhanced T1-weighted image showing a ring enhancing lesion on the left side compressing the left lateral ventricle making it impossible to appreciate ependymal enhancement
Flair signal proximity to ventricular ependyma was present in 50.67% (n = 38) patients [Figure 5] and absent in 49.33% (n = 37) patients [Figure 6]. Among HGGs, 45.61% (n = 26) in Grade IV and 19.29% (n = 11) in Grade III showed FLAIR signal proximity to ependyma [Tables 6 and 7].
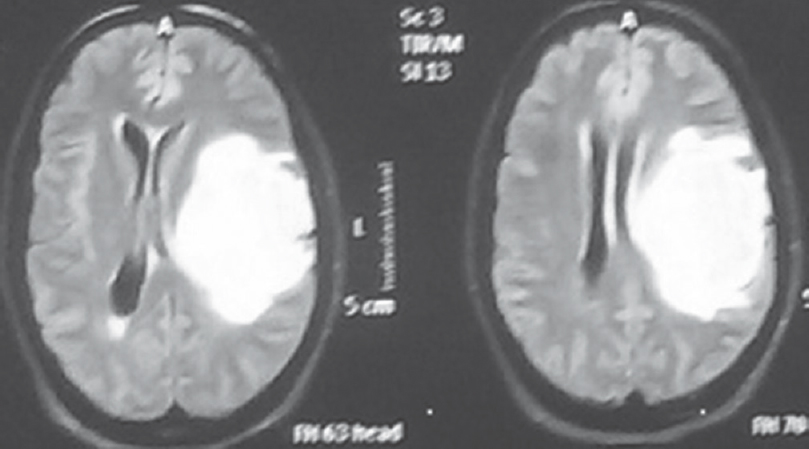
- Fluid-attenuated inversion recovery signal proximity to ependyma present
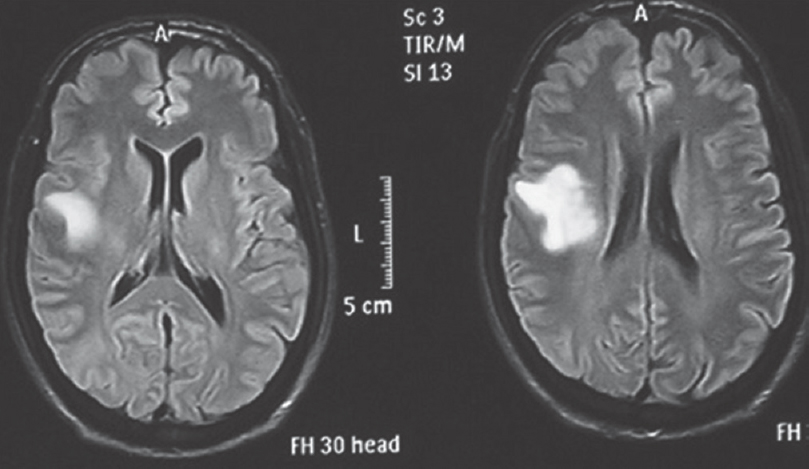
- Fluid-attenuated inversion recovery signal proximity to ependyma absent
DISCUSSION
Gliomas have been classified by the WHO into four grades. Grade I shows low proliferative potential and the possibility of cure following surgical resection alone. Grade II defines diffusively infiltrative tumors with cytological atypia alone, for example, diffuse astrocytoma. Grade III has atypia, anaplasia, and mitotic activity, for example, anaplastic varieties of astrocytoma, ependymoma, and oligoastrocytoma. Grade IV tumors additionally show microvascular proliferation and necrosis, for example, GBM.
LGGs are typically hypointense on T1, hyper on T2 relative to white matter, contrast enhancement may be absent or, at best, mild. Grade III astrocytoma often invade structures without destroying them, causing their ill-defined borders. The mass is inhomogeneous and bright on T2-weighted images. Surrounding edema and/or tumor infiltration is usually appreciated. Enhancement is usually seen. Grade IV tumors (GBM) are usually discovered as bulky disease, and necrosis is a hallmark of this grade. These lesions usually enhance peripherally, in a nodular and irregular manner, and they cause a large amount of mass effect and edema.[6] These tumors often cross the corpus callosum, giving them a typical butterfly shape. Areas of hemorrhage and necrosis are common.
Treatment of HGGs consists of maximal safe surgical resection followed by radiotherapy (RT) with temozolomide chemotherapy.[8] GBM carries a poor prognosis with median survival of 12–14 months. Prognosis and response to standard therapy with resection, radiation, and chemotherapy are highly variable suggesting heterogeneous nature of this deadly disease.[4] Recent evidences show that heterogeneity seen in GBM may be related to the cells of origin, NSCs in SVZ. The SVZ is composed of four layers. Layer 1 is the innermost layer, composed of ependymal cells. Layer 2 contains astrocytic processes connecting ependymal cells with astrocytes.[3] Layer 3 consists of astrocytic cell bodies that have been implicated as potential sources of multipotent stem cells. Layer 4 has myelin and separates Layer 3 from underlying brain parenchyma. Prior studies suggest that GBM adjacent to the SVZ are more likely to manifest the highly invasive and multifocal phenotype and may be associated with increased recurrence rate and morbidity.[4910111213] Hence, this subset of GBM or the gliomas related to SVZ should be treated more aggressively early in their disease from therapy specifically targeting the SVZ.
Patient age, performance status, extent of resection, and chemo-RT are long established prognostic factors in GBM patients.[14] Research progress on molecular aspect of GBM has revolutionized our understanding of the disease, and it is recently learned that prognosis correlates with molecular characteristics (Hyper methylation of MGMT) of the tumor.[15] Because it is not practically possible to provide molecular profiling to every patient, we aim to test the association between tumor grade and the proximity to SVZ to further predict the outcome of this deadly disease[1617] and to provide a radiological tool in the form of ependymal enhancement.
In our study, based on ependymal enhancement, cases were divided into three groups, with evidence of enhancement, without evidence of enhancement, and as inconclusive. This distribution is similar to the one adopted by Kaidar-Person et al.[16] The inconclusive group was not taken into calculations regarding ependymal enhancement.
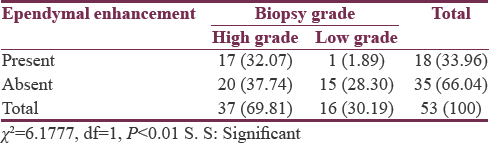
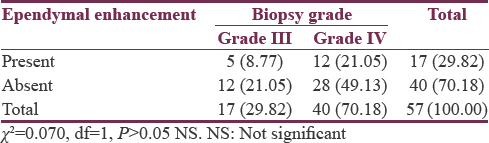
Glioma tumor grade was significantly associated with ependymal enhancement as, 32.07% (n = 17) patients of high grade and only 1.89% (n = 1) of low grade showed enhancement (P < 0.01). On further analyzing among HGGs, 8.77% (n = 5) in Grade III and 21.05% (n = 12) in Grade IV had evidence of ependymal enhancement which was not statistically significant (P > 0.05). Glioma tumor grade was significantly associated with T2 FLAIR signal proximity to the ventricular ependyma (P < 0.001). A total of 50.67% (n = 38) patients had T2 flair signal proximity to ependyma, of which 37 (49.33%) were HGGs and only 1 (1.33%) was of low grade. Among HGGs, 45.61% (n = 26) in Grade IV and 19.29% (n = 11) in Grade III showed FLAIR signal proximity to ependyma which was not statistically significant (P > 0.05).
On MRI, GBM has been observed to contain a high frequency of T2-weighted/FLAIR hyperintense regions contiguous with the posterior SVZ.[17] There are multiple studies show that patients with GBM in contact with SVZ show shorter survival[9101112] and more aggressive recurrence.[13] Our results also support that higher grade tumors are more closely linked to the SVZ, as a rate of ependymal enhancement was significantly high in HGGs. Our study also reflects that among HGGs, the rate of ependymal enhancement is more in GBM as compared to Grade III tumors, but it was not statistically significant. This observation shows that relationship to SVZ also holds good for Grade III tumors. These results are in accordance with prior results as shown by Smith et al.[17]
Our results demonstrate that higher tumor grade is associated with the proximity of MRI T2-weighted FLAIR signal to the ependyma. This observation also suggests that higher grade tumors are closely related to the SVZ. On further analyzing HGGs, T2 FLAIR proximity was more in GBM than Grade III tumors, but it was not statistically significant.
Contrary to this hypothesis, there are studies that failed to show the relationship and showed that pretreatment ependymal enhancement on MRI in GBM was not associated with poor survival.[16] All these observations make it more interesting and merit further investigation.
While previous studies examined relationship between GBM behavior and SVZ,[910111213] very few studies have included LGGs.[1718] There has been a study of Grade II astrocytoma showing increased tumor volume in tumors adjacent to the SVZ, suggesting that proximity to SVZ may correlate with enhanced proliferative potential[18] and even LGGs may have association with the SVZ. Our study also included LGGs. Ependymal enhancement was present in only one LGG, and further studies are needed before commenting on relationship of LGG with SVZ.
Analysis of clinical factors (other than ependymal enhancement) to predict tumor grade showed that age and KPS were significantly associated across tumor grades. Mean ± SD age was 39.39 ± 12.07 years for Grade II (n = 18), 40.18 ± 13.41 years for Grade III (n = 18), and 49.40 ± 12.79 years for Grade IV (n = 40) and was statistically significant (P <.05) between Grade II/Grade IV and Grade III/Grade IV. Mean ± SD KPS was 82.94 ± 15.25 for Grade II (n = 18), 82.94 ± 15.25 for Grade III (n = 17), and 70.00 ± 20.73 for Grade IV (n = 40) and was statistically significant between Grade II and Grade IV (P < 0.05). Both these findings were in accordance with the prior studies.[14]
The merits of our study are that it is a prospective study that attempts to describe the role of preoperative ependymal enhancement with respect to the grade of gliomas. It also includes LGGs so that role of SVZ can be studied across all the grades.
Our study has few limitations. First, it is a relatively small sample size of 75 patients, and of these, in 22 cases, ependymal enhancement was inconclusive for involvement reducing the size further.
CONCLUSION
This study shows that preoperative ependymal enhancement and FLAIR signal proximity to ependyma are associated with HGGs. It can be helpful in predicting the tumor grade preoperatively, and by including LGG in the study, the role of SVZ in the heterogeneous disease process of glioma can be studied as a whole, not only in the GBM. Further studies oriented toward both LGGs and HGGs are needed to see the utility of ependymal enhancement and whether it can be established as a radiological tool to predict the grade so that more intensive therapeutic options can be applied to this subset of patients and better results in terms of tumor-free period and survival can be obtained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The role of the subependymal plate in glial tumorigenesis. Acta Neuropathol. 1977;40:63-71.
- [Google Scholar]
- The human brain subventricular zone: Stem cells in this niche and its organization. Neurosurg Clin N Am. 2007;18:15-20, vii.
- [Google Scholar]
- Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424-9.
- [Google Scholar]
- MRI of high-grade glial tumors: Correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology. 1997;39:348-50.
- [Google Scholar]
- Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27:65-73.
- [Google Scholar]
- MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26:2466-74.
- [Google Scholar]
- Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
- [Google Scholar]
- Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219-24.
- [Google Scholar]
- Stem cell associated gene expression in glioblastoma multiforme: Relationship to survival and the subventricular zone. J Neurooncol. 2010;96:359-67.
- [Google Scholar]
- Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol. 2011;104:261-9.
- [Google Scholar]
- Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15:91-6.
- [Google Scholar]
- Glioblastoma multiforme: Relationship to subventricular zone and recurrence. Neuroradiol J. 2013;26:542-7.
- [Google Scholar]
- Prognosis factors of survival time in patients with glioblastoma multiforme: A multivariate analysis of 340 patients. Acta Neurochir (Wien). 2007;149:245-52.
- [Google Scholar]
- Temozolomide resistance in glioblastoma cell lines: Implication of MGMT, MMR, P-glycoprotein and CD133 expression. PLoS One. 2015;10:e0140131.
- [Google Scholar]
- The clinical significance of ependymal enhancement at presentation in patients with malignant glioma. Rambam Maimonides Med J. 2015;6(4):e0039.
- [Google Scholar]
- Use of preoperative FLAIR MRI and ependymal proximity of tumor enhancement as surrogate markers of brain tumor origin. J Clin Neurosci. 2015;22:1397-402.
- [Google Scholar]
- World Health Organization Grade II gliomas and subventricular zone: Anatomic, genetic, and clinical considerations. Neurosurgery. 2011;68:1293-8.
- [Google Scholar]






