Translate this page into:
The QT dispersion and QTc dispersion in patients presenting with acute neurological events and its impact on early prognosis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
To find out and investigate whether the QT dispersion and QTc dispersion is related to type and prognosis of the acute stroke in patients presenting within 24 h of the onset of stroke.
Settings and Design:
This was a observational study conducted at Mahatma Gandhi Hospital, Dr. SN. Medical College, Jodhpur, during January 2014 to January 2015.
Subjects and Methods:
The patients presented within 24 h of onset of acute stroke (hemorrhagic, infarction, or transient ischemic event) were included in the study. The stroke was confirmed by computed tomography scan and magnetic resonance imaging. Patients with (i) altered sensorium because of metabolic, infective, seizures, trauma, or tumor; (ii) prior history of cardiovascular disease, electrocardiographic abnormalities’ because of dyselectrolytemia; and (iii) and patients who were on drugs (antiarrhythmic drugs, antipsychotic drugs, erythromycin, theophylline, etc.,) which known to cause electrocardiogram changes, were excluded from the study. National Institute of Health Stroke Score (NIHSS) was calculated at the time of admission and Modified Rankin Scale (MRS) at the time of discharge. Fifty age- and sex-matched healthy controls included.
Statistical Analysis Used:
Student's t-test, ANOVA, and area under curve for sensitivity and specificity for the test.
Results:
We included 52 patients (male/female: 27/25) and 50 controls (26/24). The mean age of patients was 63.17 ± 08.90 years. Of total patients, infarct was found in 32 (61.53%), hemorrhage in 18 (34.61%), transient ischemic attack (TIA) in 1 (1.9%), and subarachnoid hemorrhage in 1 (1.9%) patient. The QT dispersion and QTc dispersion were significantly higher in cases as compare to controls. (87.30 ± 24.42 vs. 49.60 ± 08.79 ms; P < 0.001) and (97.53 ± 27.36 vs. 56.28 ± 09.86 ms; P < 0.001). Among various types of stroke, the mean QT dispersion and QTc dispersion were maximum and significantly higher in hemorrhagic stroke as compared to infarct and TIA (P < 0.001). The mean QT dispersion and QTc dispersion was found significantly high in nonsurvivors (n = 16) as compared to survivors group (n = 36) (P < 0.05). The mean QT dispersion was directly correlated with the NIHSS and functional outcome score MRS. Patients with greater QT and QTc dispersion having high NIHSS had poor prognosis.
Conclusion:
We concluded that patients presenting with acute neurological events having increased QT dispersion and QTc dispersion is related to high mortality and poor functional outcomes on hospital discharge and if the values of dispersion score are very high we can predict for hemorrhagic stroke.
Keywords
Acute stroke
hemorrhage
infarct
prognosis
QT dispersion
Introduction
Stroke is the second most common cause of death in the world, after Coronary Artery Disease-related deaths.[1] It is also a very important single cause responsible for morbidity of disease. The central nervous system (CNS) has an important role in regulation of cardiac activity and vasomotor tones.[234] The lesions of the CNS frequently lead to disturbance of cardiovascular system (CVS) and other autonomic functions.[5] The manifestations of such type of autonomic dysregulation are loss of heart rate variability and various electrocardiogram (ECG) changes.[67] The sympathetic tone is increased in acute ischemic stroke, which leads to elevated levels of circulating catecholamines and responsible for cardiac complications including arrhythmias, ECG changes, and ischemic heart damage, and associated with the poor prognosis of the disease.[89] The cardiovascular autonomic failure may also result from impaired parasympathetic functions in the acute stroke.[10] The cardiovascular autonomic dysregulation can lead to increased QT dispersion. It is almost a direct measure of the heterogeneity of myocardial repolarization. The QT dispersion varies with the subtype and location of the cerebral lesion. The QT dispersion has been used as a predictor of the adverse outcomes in various cardiac and noncardiac states.[1112131415] Lazar et al.[16] found increased QT dispersion on admission ECG in patients presenting with acute neurological events, this was significantly related to hospital mortality and poor functional outcome on discharge. Our study was aimed to find out the relation of QT dispersion and QTc dispersion with stroke subtype and its prognostic importance.
Subjects and Methods
This was an observational case control study of 52 patients. This study was conducted in the department of the medicine at Mahatma Gandhi Hospital Jodhpur, Rajasthan, India, over a period of 1 year (March 2014–March 2015). The study was approved by the Ethical committee of the Medical College. Detailed personal history and examination were done using a standard performa.
Inclusion criteria
The patients of acute cerebrovascular accident (CVA) within 24 h of onset were included in the study. CVA included ischemic stroke, hemorrhagic stroke, subarachnoid hemorrhage (SAH), and transient ischemic events. The diagnosis of CVA was confirmed by computed tomography or magnetic resonance imaging.
Exclusion criteria
-
Patients with prior history of cardiovascular disease (ischemic, valvular heart disease, cardiac arrhythmia, or cardiomyopathy)
-
Altered mental status because of metabolic disturbances, seizures, space occupying lesion, and infections
-
Patient taking medication known to affect repolarisation parameter on the ECG, i.e., digoxin, antiarrythmic drugs, phenothiazines, tricyclic antidepressant, lithium carbonate, erythromycin, theophylline, levodopa, etc.
-
And Patients whose ECG reveals a bundle branch block pattern, sick sinus, and other changes related to disease other than CVA.
The QT dispersion and QTc dispersion were calculated at the time of the admission of patients on a 12 lead ECG. QT dispersion is the difference between maximum and minimum QT interval on the 12 lead ECG. The QTc dispersion is the difference between the maximum and minimum QTc interval on the 12 lead ECG.[17] The routine hematological, biochemistry and electrolytes tests were checked. National Institute of Health Stroke Score (NIHSS) was calculated at the time of the admission, and clinical outcome was recorded at the time of the discharge of the patients and graded according to the Modified Rankin Scale (MRS).[1819]
Control group
An age- and sex-matched 50 healthy controls (male/females - 25/25) were included in the study for comparison of the baseline QT dispersion and QTc dispersion on 12 lead ECG.
Statistical analysis
The parametric data are expressed as mean value ± standard deviation (SD) and categorical variables as percentages. The Chi-square test was used for the comparison of dichotomous variables and the Student's t-test for continuous variables. One-way ANOVA was used to test differences on multiple levels by a single factor (independent) variable. The sensitivity and specificity of the QT dispersion and QTc dispersion for mortality were calculated by the area under curve (AUC). The P < 0.05 were considered statistically significant. All data were analyzed using the SAS 8.0 statistical package.
Results
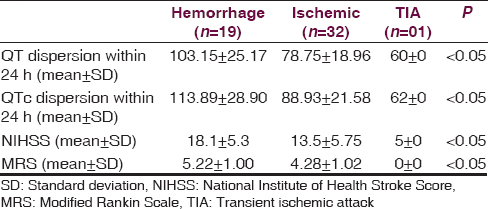
The study included 52 patients (male/female = 27/25) with acute stroke who fulfilled inclusion criteria. The mean ± SD ages for the study and control group was 63.17 ± 8.9 years and 63.87 ± 7.5 years, respectively (P = 0.08). There were 32 (66.19%) ischemic, 18 (24.64%) hemorrhagic, 1 (9.15%) transient ischemic attack (TIA), and 1 (9.15%) SAH strokes. The most common risk factor in our patients was hypertension in 68.30% followed by diabetes mellitus in 28.87% and dyslipidemia in 23.94% patients. The mean QT dispersion and mean QTc dispersion were significantly higher in patients of acute stroke as compared to healthy controls (87.30 ± 24.42; vs. 49.60 ± 08.79 ms; P < 0.05) and (97.53 ± 27.36 vs. 56.28 ± 09.86 ms P < 0.05) [Table 1]. The mean QT dispersion and QTc dispersion values were maximum for patients with intracerebral hemorrhage (ICH) (103.15 ± 25.17 ms; 113.89 ± 28.90) that was followed by ischemic stroke (78.75 ± 18.96 ms; 88.93 ± 21.58 ms) and TIA (60 ± 00 ms; 62 ± 00 ms). The difference in the values of QT dispersion and QTc dispersion among various types of stroke was statistically significant (P < 0.05) [Figure 1 and Table 2].

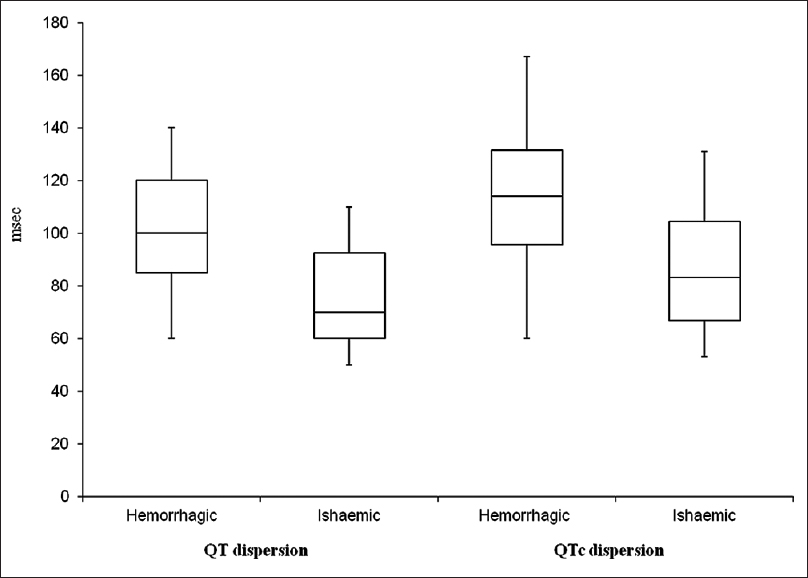
- Box diagram of QT dispersion and QTc dispersion in hemorrhagic and ischemic stroke patients
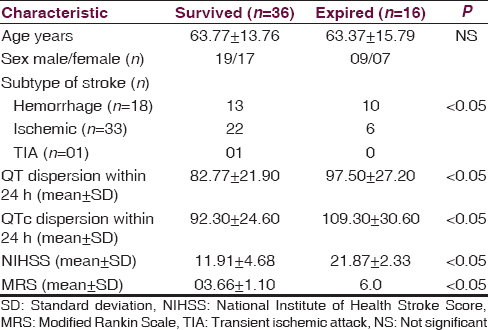
A total, 16 (21.9%) patients expired. Of the 16 expired patients, 07 patients had ischemic, and 9 had hemorrhagic stroke. The demographic and clinical difference between patients who expired and those who survived are mentioned in Table 3. The QT dispersion and QTc dispersion were significantly higher in patients who expired after stroke than in patients who survive (97.50 ± 27.20 vs. 82.77 ± 21.90 ms P < 0.005) and (109.30 ± 30.60 vs. 92.30 ± 24.60 ms, P < 0.001). The expired patient also had significantly higher value of NIHSS as compared to survived group [Table 3 and Figure 2]. The QT dispersion and QTc dispersion within the 24 h of onset of stroke were also compared with the NIHSS calculated at the time of the presentation of the patients and with MRS which was calculated at the time of the discharge. The QT dispersion and QTc dispersion values were positively correlated with the severity of the patients at the time presentation on NIIHSS within 24 h of onset of stroke (P < 0.05) Mean QT dispersion and mean QTc dispersion value significantly correlated with poor clinical outcome as determined with MRS. This correlation was statistically significant (P < 0.05). There was statistically significant increment in the QT dispersion and QTc dispersions with change in the group of NIHSS. The NIHSS groups were made according to the severity as NIHSS 0-7 mild, NIHSS 8-15 moderate, and NIHSS > 15 was considered as severe [Table 4]. The sensitivity and specificity of the QT dispersion and QTc dispersion for mortality at the hospital were calculated by AUC. The QT dispersion was 62.5% sensitive and 69.44% specific on cut-off value at > 90 ms by AUC. The QTc dispersion was 62.5% sensitive and 72.25% specific on cut-off value at >105 ms [Table 5 and Figure 3].
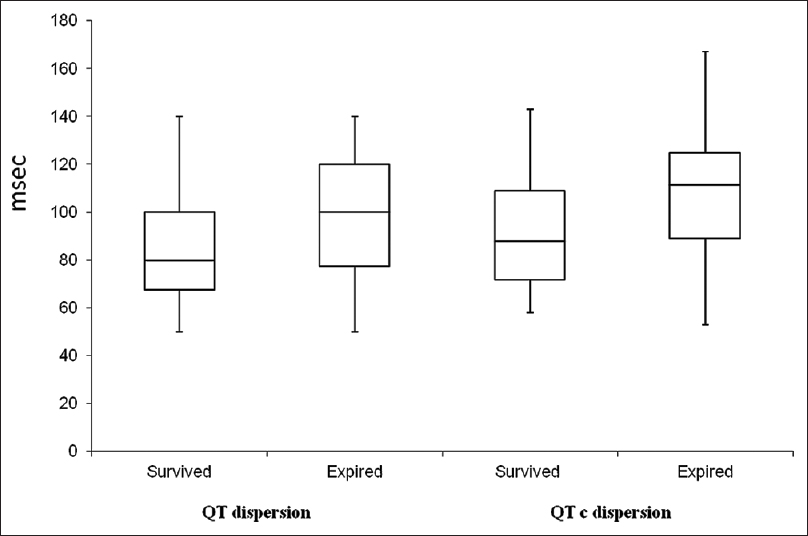
- Box diagram of QT dispersion and QTc dispersion in nonsurvived and survived patients

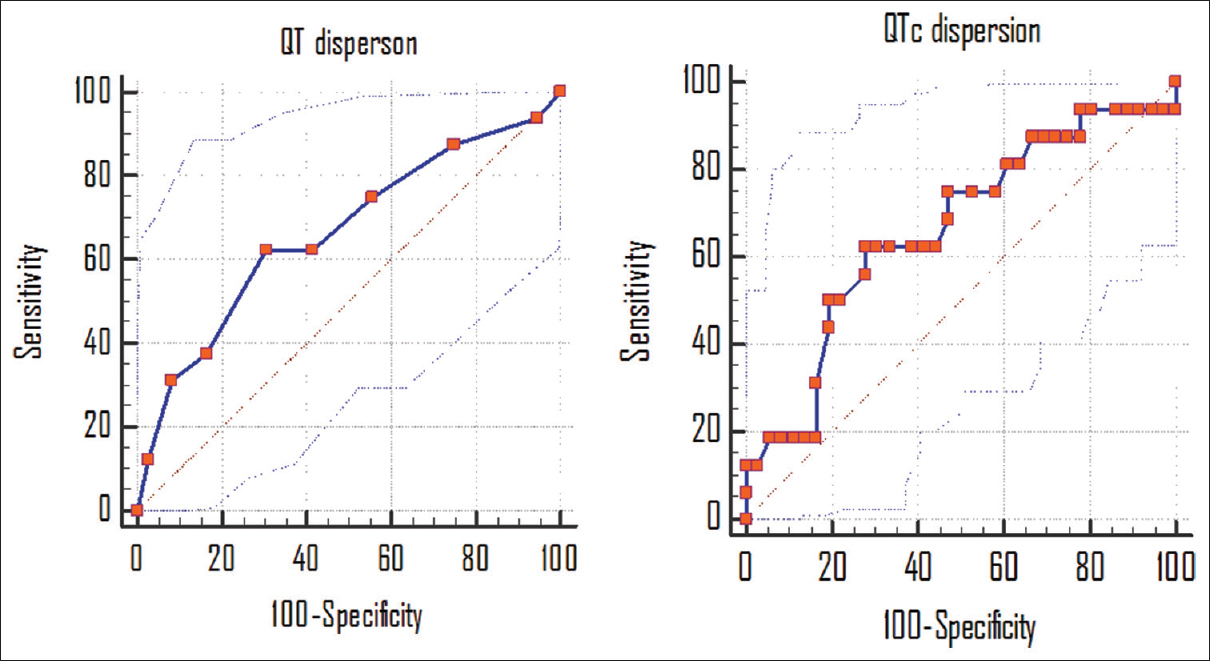
- Sensitivity and specificity of QT dispersion and QTc dispersion in the prognosis of the stroke patients
Discussion
There are evidence for an interaction between the CNS and the CVS during acute CVAs. The autonomic and ECG changes after acute stroke in patients without having any cardiac disease are common, these are considered because of dysautonomic activity or altered autonomic regulation of the CVS. The common ECG change encountered in the acute stroke are increased QT interval, increased QT dispersion, ST segment changes, T wave inversion, pathological Q wave, supraventricular and ventricular tachyarrhythmias in the absence of the autopsy-proven cardiac diseases or which are not related to ischemic heart disease.[2021222324] Over the past decade, the QT dispersion and QTc dispersion have been used as a prognostic indicator in the patients with cardiovascular disease at risk for ventricular tachyarrhythmias and sudden death. These autonomic and ECG changes depend on the type and location of the stroke, and adversely affect the outcome of the disease. It has been found that patients with right stroke involving insular cortex are more susceptible to develop cardio-autonomic dysfunction. The sympathetic activity is found to be significantly higher in insular than in noninsular infarction. This also suggests a neurogenic rather than primary cardiac-autonomic control.[25262728293031] Our study aimed to find out the predictive role of QT dispersion and QTc dispersion in various type of stroke and its prognostic significance in the mortality and morbidity of the disease. In our studies the QT dispersion and QTc dispersion values in healthy controls were 49.60 ± 8.79 ms and 56.28 ± 09.86 ms, respectively. Alabd et al.[26] found QT dispersion and QTc dispersion of 43 ± 5 ms and 48 ± 5 ms in controls. Afsar et al.[8] studied 36 patients of acute stroke without cardiac disease and recorded QT dispersion and QTc dispersion at first 24 h and at after 72 h. The QT dispersion, corrected QT dispersion, and automated QT dispersion were significantly increased in the 24 h-ECG compared with the 72 h-ECG (mean 60 ms vs. 40 ms, P < 0.005, 56 v/s 36 ms, P < 0.001; and 50 vs. 34 ms, P < 0.005, respectively). However, QT dispersion in the 72 h-ECG was similar to QT dispersion in the control group. They also found significantly greater QTc dispersion in the 24 h-ECG corrected in patients with large infarcts and large hemorrhages (mean ± SD, 70 ± 20 vs. 51 ± 20 ms, P < 0.05). Our findings were also supported by the Afsar et al.,[8] who also found significantly greater values of QT dispersion in ICH patients.
Lazar et al.[16] found significantly higher value of QT dispersion in patients with ICH as compared to infarct and TIA (70 ± 15 ms vs. 53 ± 27 ms vs. 48 ± 31 ms, respectively), among studied 140 patients of CVA and TIA. The increasing QT dispersion was associated with lower functional outcomes on all 3 scales and with higher mortality. The QT dispersion was higher in patients with congestive heart failure and with carotid disease as compared to those without. Several studies have evaluated the relationship between the sympathetic activity and QT dispersion and QTc dispersion. In a study done by Randell et al.[32] in patients with SAH, found greater QT dispersion directly correlated with the plasma concentrations of 3,4-dihydroxyphenylglycol, which is a metabolite of norepinephrine. Myers et al.[33] found significantly higher levels of plasma norepinephrine, in patients with stroke, and explained the cardiac abnormalities in cerebral infarction by increased sympathetic activity. Direct evidence linking reduced parasympathetic control and increased QT dispersion is lacking despite reduced parasympathetic controlled heart rate variability in patients with ischemic stroke.[24] Although carotid baroreceptor dysfunction has been demonstrated after acute stroke, baroreceptor function is also impaired in patients with congestive heart failure and carotid artery disease.[34] Therefore, a number of reasons exist for baroreceptor dysfunction and the specific mechanism by which acute neurological events increase QT dispersion remains unknown.
In our study, we found considerable variability in the QT dispersion and QTc dispersion on admission ECG in patients presenting with acute stroke within 24 h. The QT dispersion and QTc dispersion contributes an important predictor of stroke type and prognostic factor as both values were greater in patients with ICH as compared to those with infarct and TIA. Higher QT dispersion and QTc dispersion values were associated with greater hospital mortality and strongly related with lower functional outcomes on MRS scales of functional disability. The variability in the QT dispersion and QTc dispersion in our patients can be explained by centrally mediated sympathetic hyperactivity, reduced cardiac parasympathetic innervations, and abnormal baroreceptor function. The QT dispersion has been proposed to reflect the heterogeneity of ventricular repolarization, which not only reflects underlying structural heart disease but also cardiac regulation by the sympathetic nervous system.
Conclusion
We concluded that patients presenting with acute neurological stroke having increased QT dispersion and QTc dispersion on 12 lead ECG at the time of presentation have more chances of hemorrhagic stroke and associated with higher hospital mortality, high NIHSS, poor functional outcome and high disability on MRS. However, it appears that altered cardiac neuroregulation can increase heterogeneity of ventricular repolarization. Hence, the ECG could be a useful tool for the prediction of impact on stroke prognosis in the patients of CVA who present within the 24 h of the onset.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- WHO: The Top 10 Causes of Death Online Address. 2011. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index.html
- [Google Scholar]
- Cardiovascular regulation and lesions of the central nervous system. Ann Neurol. 1985;18:1-13.
- [Google Scholar]
- Neurogenic cardiac effects of cerebrovascular disease. Curr Opin Neurol. 1994;7:20-4.
- [Google Scholar]
- Circadian rhythm of heart rate variability is reversibly abolished in ischemic stroke. Stroke. 1997;28:2150-4.
- [Google Scholar]
- Acute stroke increases QT dispersion in patients without known cardiac diseases. Arch Neurol. 2003;60:346-50.
- [Google Scholar]
- Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833-8.
- [Google Scholar]
- QT dispersion: An indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342-4.
- [Google Scholar]
- QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327-9.
- [Google Scholar]
- QTc dispersion predicts cardiac mortality in the elderly: The Rotterdam Study. Circulation. 1998;97:467-72.
- [Google Scholar]
- Stroke-associated pathological sympathetic activation related to size of infarction and extent of insular damage. Cerebrovasc Dis. 1995;5:381-5.
- [Google Scholar]
- The prognostic value of QT dispersion in patients presenting with acute neurological events. J Invasive Cardiol. 2003;15:31-5.
- [Google Scholar]
- Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Arch Neurol. 2004;61:1677-80.
- [Google Scholar]
- Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243-6.
- [Google Scholar]
- QT dispersion after subarachnoid haemorrhage. J Neurosurg Anesthesiol. 1999;11:163-6.
- [Google Scholar]
- Cerebrogenic cardiac arrhythmias. Cerebral electrocardiographic influences and their role in sudden death. Arch Neurol. 1990;47:513-9.
- [Google Scholar]
- Supraventricular tachycardia in patients with right hemisphere strokes. Stroke. 1992;23:362-6.
- [Google Scholar]
- The electrocardiogram in stroke: Relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253-9.
- [Google Scholar]
- Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30:1307-11.
- [Google Scholar]
- QT dispersion on ECG in acute ischemic stroke and its impact on early prognosis. Neurosciences (Riyadh). 2008;13:366-9.
- [Google Scholar]
- QT interval dispersion pattern in patients with acute ischemic stroke: Does the site of infarction matter? Int J Angiol. 2009;18:177-81.
- [Google Scholar]
- Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry. 2005;76:269-71.
- [Google Scholar]
- Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke. 2005;36:1710-5.
- [Google Scholar]
- Prolongation of the frequency-corrected QT dispersion following cerebral strokes with involvement of the insula of Reil. Eur Neurol. 1999;42:190-3.
- [Google Scholar]
- Importance of lead selection in QT interval measurement. Am J Cardiol. 1988;61:83-7.
- [Google Scholar]
- QT dispersion after subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:163-6.
- [Google Scholar]
- Cardiac baroreceptor sensitivity is impaired after acute stroke. Stroke. 1997;28:1671-6.
- [Google Scholar]






