Translate this page into:
Unilateral Papilledema in Cerebral Venous Sinus Thrombosis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the majority of patients with raised intracranial pressure, the papilledema is bilateral. Unilateral papilledema is rare in conditions causing intracranial hypertension, and it has been described in Foster–Kennedy syndrome and in some cases of idiopathic intracranial hypertension. It has never been reported in cerebral venous thrombosis. We report a young lady presenting with features of subacute onset of headache with seizures, on evaluation she had superior sagittal and bilateral lateral sinus thrombosis. The risk factors found on evaluation were Vitamin B12 deficiency and hyperhomocysteinemia. On optic fundus examination, she had swollen optic disc on the right side with normal fundus on the left side, confirmed with the orbital ultrasound B-scan and optic coherence tomography. Her magnetic resonance imaging showed features of raised intracranial pressure with thrombosis of the superior sagittal and bilateral lateral sinus thrombosis. She was treated with anticoagulation (heparin followed by oral anticoagulants), antiedema measures, and vitamin supplementation for hyperhomocysteinemia. She improved over time and was asymptomatic during follow-up. We discuss the possible mechanisms described in the literature for unilateral papilledema. This report highlights the need for carefully performing bilateral fundus examination so as not to miss the vision or life-threatening causes of a headache.
Keywords
Cerebral venous sinus thrombosis
intracranial pressure
optic fundus
papilledema
INTRODUCTION
Papilledema is a clinical sign observed in the neurological practice due to etiologies with an underlying mechanism of raised intracranial pressure. Careful observation of the optic fundus to look for this important sign is of utmost importance as it may indicate serious intracranial pathology. It is not only looking for subtle features of papilledema but also to examine bilaterally as the absence of papilledema in one eye may preclude examination of another eye. This may be in many instances on the theoretical reasoning of equal expression of raised intracranial pressure in both the eyes (a belief held by many physicians, and thus missing unilateral papilledema!). There have been reports of unilateral papilledema in idiopathic intracranial hypertension and other intracranial disorders.[123] We report an interesting patient who had unilateral papilledema due to cerebral venous sinus thrombosis (CVT) and also look at the possible mechanisms proposed to explain the unilaterality of this sign in conditions with raised intracranial pressure.
CASE REPORT
A 16-year-old Kannada-speaking Indian female presented with 3-week history of headache, and multiple episodes of vomiting. One day before her presentation to our hospital, she developed three episodes of generalized tonic–clonic seizures with preserved sensorium in between the attacks. She denied any double vision, facial weakness, tinnitus, speech, or swallowing difficulties. There was no history of fever, ear pain, or ear discharge. She was otherwise healthy without any prior medical or neurological illness. She was not on any long or short-term medications before her symptoms started. She was unmarried and had regular menstrual cycles. Her diet predominantly involved vegetarian menus, rich in carbohydrates, poor in protein, and dairy products.
On examination, her weight was 53 kg, height 160 cm, and body mass index = 20.7 kg/m2. She had knuckle hyperpigmentation with mild pallor. No organomegaly was detectable clinically. She was conscious oriented with normal higher mental functions and language assessment. Her vision for near and far objects was normal (6/6) without correction and so was her color vision in both eyes. Her visual fields by confrontation method were normal. Eye movements were normal and full range. Fundus examination showed swelling of her right optic nerve head with disc elevation, obliteration of the cup, blurring of the disc margins, and peripapillary halo with fundal hemorrhage with no major vessel obscurations on the right side (Frisen grade-2). The left eye fundus was normal [Figure 1a and b]. The veins appeared congested in the right fundus, the left side being normal. Rest of the neurological examination was normal.
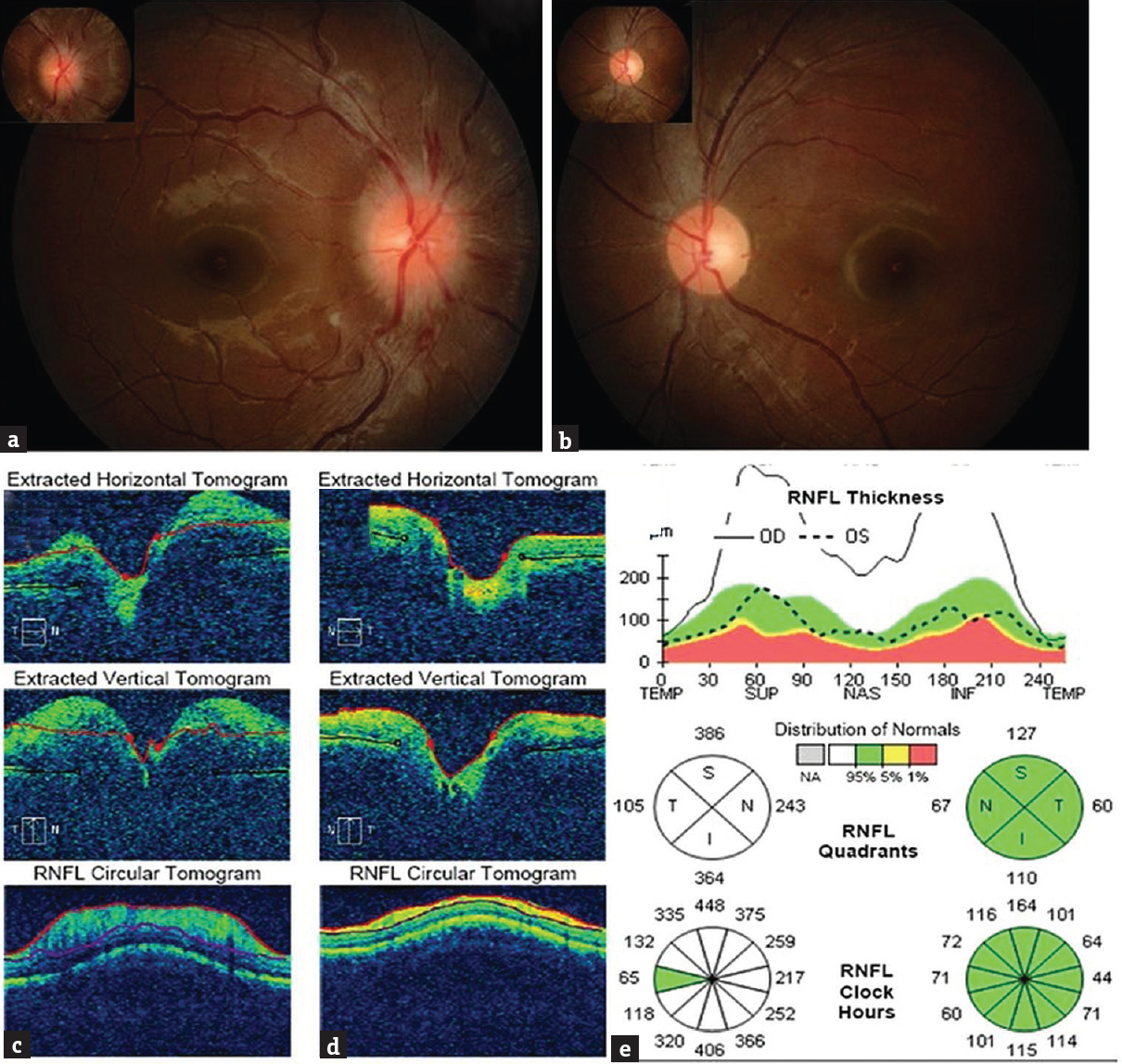
- Fundal pictures of both the eyes showing the right eye with swollen optic disc (a), left eye disc being normal (b). Optic coherence tomography of the right (c) and left (d) eye including a graphical representation of retinal nerve fiber thickness (e) in each quadrant of each optic nerve confirming the increased nerve fiber thickness and swelling due to edema on the right side
Her investigations included complete blood count, erythrocyte sedimentation rate, peripheral smear, liver/renal and thyroid functions tests, fasting glucose, lipid profile, antinuclear antibody, antiphospholipid antibody screening, serum homocysteine levels, Vitamin B12 levels, folate levels, and serum antibodies for human immunodeficiency virus by enzyme-linked Immunosorbent assay. She had dimorphic anemia (hemoglobin - 9.7 g/dl, normal 12–16 g/dl in females) with hypersegmented neutrophils, hyperhomocysteinemia (26 μmol/L, normal range 5–20 μmol/L), and low Vitamin B12 (<50 pg/ml, normal range-180–900 pg/ml) levels; other investigations were normal. Her brain imaging with computed tomographic scan (plain and contrast) (Philips Brilliance/Netherland/2010) showed hyperdense superior sagittal sinus with filling defect and similar findings in bilateral lateral sinuses [Figure 2a and e] with the right temporal hypodensity suggestive of extensive CVT. Magnetic resonance imaging (MRI) with venography (Siemens 3 Tesla/Erlangen Germany/2014) also showed thrombosis of the superior sagittal sinus and bilateral lateral sinus with parenchymal lesion [Figure 2b and f]; additionally, there was partial empty sella, prominence of perioptic spaces, tortuosity of the right optic nerve, and protrusion of the right papilla suggesting raised intracranial pressure [Figure 2b, d, g, and h]. No obvious defect in the perioptic sheath was noted in the MRI. Protrusion of the right papilla was confirmed with orbital ultrasound B-scan (Philips/Netherland/2014). We did optic coherence tomography (OCT) both the eyes to detect any subclinical papilledema in the left eye. There was significant disc elevation in the right eye and normal disc in the left eye. Nerve fiber layer (NFL) thickness was increased on the right side being normal on the left [Figure 1c–e]. Intraocular pressure (IOP) was normal bilaterally (14 mm Hg). Her visual fields by perimetry showed mildly enlarged blind spot in the right eye whereas the left was normal. We did not perform cerebrospinal fluid examination as the patient had extensive sinus thrombosis with the right temporal parenchymal lesion and imaging showing futures of raised intracranial pressure. It would not have added to the management of our patient, and we were worried about the remote possibility of worsening of her clinical condition due to brain herniation. She was treated for cerebral CVT with unfractionated heparin 5000 units 6th hourly subcutaneously for 10 days followed by oral anticoagulation (acenocoumarol loading of 4 mg/day followed by maintenance dose of 1.5 mg/day once day),[4] antiedema measures (mannitol 50 ml 6th hourly intravenously for 5 days and tapered gradually over next 3 days and oral glycerol 20 ml orally 4 times/day for 10 days), anticonvulsants (phenytoin sodium 200 mg/day in two divided doses), Vitamin B12 supplementation (1 mg intravenous injection for 7 days and 1mg intravenously once a week for 1 month followed by oral 1 mg/day tablets), pyridoxine (10 mg/day), and tablet folic acid 5 mg/day, iron supplementation (tablet ferrous sulfate 60 mg 3 times/day). Subsequently, maintenance doses of iron and vitamin supplementations were continued after 3 months. She is being followed up in the outpatient department monthly from the past 5 months. Her repeat eye examination showed resolved right optic fundus edema. We have planned to repeat her serum homocysteine and Vitamin B12 levels at 6 months and if they are normal, we will stop anticoagulation. She will continue to receive anticonvulsants for total duration of 1 year, followed by tapering and stopping if there is no seizure recurrence.
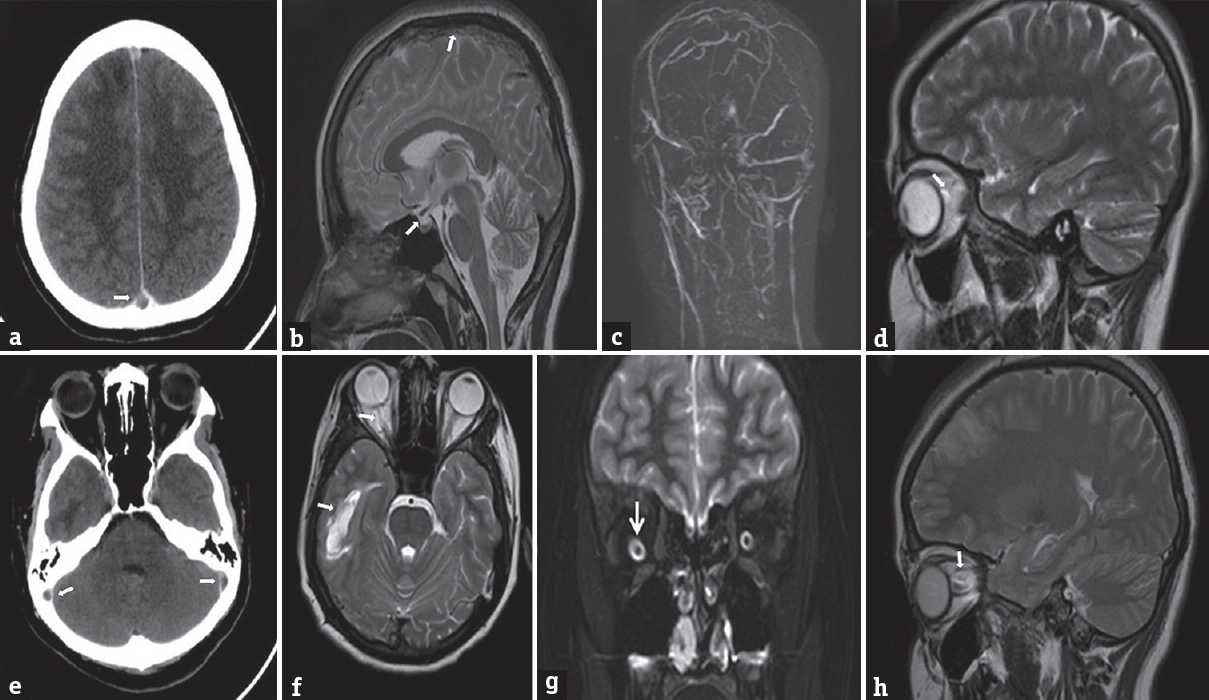
- Postcontrast computerized tomographic scan of the brain showing (a) (superior sagittal), (e) (bilateral lateral sinus) thrombosis in the form of filling defects (arrows). Magnetic resonance imaging T2 Sagittal scan (b) showing thrombosis of superior sagittal sinus and partial empty sella (arrows). Magnetic resonance venogram showing nonvisualization of superior sagittal and bilateral lateral sinuses (c). Magnetic resonance imaging brain sagittal (d and h) showing prominent and tortuous right optic sheath (arrow) more pronounce on coronal T2 magnetic resonance imaging image (g) (arrow). Magnetic resonance imaging T2 axial (f) shows right temporal parenchymal lesion (arrow)
DISCUSSION
The reported occurrence of papilledema in patients with CVT is about 25%–75% in the literature and it is bilateral.[56] Our literature search on PubMed database (with search terms papilledema and or/with cerebral venous thrombosis) did not show any mention of unilateral papilledema in CVT. Among the secondary causes of headache, CVT is an important and often overlooked condition, and if not recognized and treated early, it can be fatal also in some patients. Headache is the most common manifestation of CVT and present in 80–90% of the patients; sometimes, it may be the only clinical manifestation of the disease and multiple mechanisms have been reported for its occurrence.[7] There are no population-based studies from India describing the incidence of CVT. A cross-sectional epidemiological study from the Netherlands showed an incidence of CVT to be 1.32/100,000/year.[8] An autopsy series found that CVT accounted for almost 10% of all strokes in India.[9] The mortality of CVT reported to be 30%–50% in earlier studies, but recent studies show better outcome and a large study from India showed the mortality being 12.9% (in the cohort of 612 patients).[10] Our case demonstrates the unusual finding of unilateral papilledema in CVT. It also highlights the fact that bilateral examination of the fundus is mandatory, whenever evaluating a suspected patient with raised intracranial pressure. These rare cases may provide a window of opportunity to peek into the possible mechanism of these unusual presentations.
The term “papilledema” indicates swelling of the optic discs secondary to increased intracranial pressure. Various mechanisms have been proposed to describe the pathogenesis of the papilledema in intracranial hypertension. Increased pressure around optic nerve causes axoplasmic flow stasis in the prelaminar nerve fibers and also there is venous congestion due to compression of draining veins which are also compressed by axonal swelling, interstitial edema resulting in disc edema.[11] As subarachnoid space is continuous around the brain and extends around the optic nerve as perioptic space, papilledema is seen bilaterally though can be asymmetrical on few occasions. Unilateral papilledema is rare in patients with raised intracranial pressure and has been sporadically reported in idiopathic intracranial hypertension,[123] intracranial tumors (Foster–Kennedy syndrome [FKS]),[12] ocular-orbital pathologies (Optic sheath tumor),[13] after vaccine administration,[14] tumor infiltration[15] and in patients with differences in IOP after trabeculectomy surgery for the eyes.[16]
It is important to distinguish unilateral papilledema from unilateral ocular conditions presenting with pseudopapilledema before labeling and evaluating the patient for papilledema which may require invasive tests (like lumbar puncture) as well as long-term management. Common conditions causing pseudopapilledema are optic disc drusen, congenital disc anomalies, hyperopic discs in young children, and myelinated nerve fibers, persistent hyaloid vessels, monocular neuropathies due to arteritic, neuritic, and nonarteritic optic neuropathy.[17] Features that aid in identifying true papilledema from pseudo papilledema include thickening and grayness of the peripapillary NFL, telangiectatic disc vessels, and obscuration of branch vessels at the disc margin in the former.[18] In addition, the absence of spontaneous venous pulsations may also help to diagnose papilledema. As noted above, we have confidently ruled out the mimickers by examination and investigations.
There is a paucity of literature explaining the unilaterality of the papilledema in intracranial pathologies with raised pressure. There are few scenarios when unilateral papilledema can be seen. In patients with pre-existing optic atrophy in one eye with subsequent development of raised intracranial pressure papilledema manifests only in the normal eye (as is the case in FKS). Other explanations offered to explain unilateral papilledema in intracranial hypertension are optic sheath defects, lamina cribrosa, or venous drainage variations which do not allow transmission of raised intracranial pressure to optic disc head.[3519] Hayreh did experimental work to show that presence of optic sheath is a prerequisite for the development of papilledema.[20] In humans, a similar mechanism is hypothesized though there is no direct evidence by imaging or pathology. We did both brain, optic nerve/sheath, orbital imaging (ultrasound, CT, and MRI imaging) to look for intracranial, optic nerve/sheath, orbital, or local ocular anatomy, explaining such an observation. Direct ophthalmoscopy was also done to detect intraocular pathology which could explain unilaterality and to exclude conditions causing pseudo papilledema. In addition, we did OCT to look for any differences in NFL thickness and optic disc elevation (detect subtle features of papilledema and also to know the anatomy). We observed an asymmetry in the optic sheath prominence between two eyes in MRI, which was more marked on the right side (eye with papilledema). It may be due to edema or possible hypothesis of differences in the compliance of two optic sheaths or asymmetry in the cranial end orifice of the optic sheath (thus limiting pressure transmission to the distal optic sheath and thus each eye) may be responsible. We cannot entirely exclude lamina cribrosa variation or trabecular meshwork changes in perioptic subarachnoid space though no macrostructural differences between the two eyes were detectable (which may change the manifestation of the raised intracranial pressure in the eye as papilledema). Major venous channels of orbit were also normal. Finally, a mechanism which can satisfactorily explain the unilateral papilledema can also explain idiopathic intracranial hypertension without papilledema.[21]
CONCLUSION
Unilateral papilledema is rare in conditions causing intracranial hypertension. It has previously not been reported in the literature in patients with cerebral venous thrombosis as per our knowledge. The clinician needs to be aware of this observation to avoid missing important secondary etiologies of headache which could be potentially treatable and should do bilateral optic fundus examination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge Dr. Bakula Kashyp ophthalmologist for providing inputs on optic fundal pictures and OCT pictures.
REFERENCES
- Asymmetric papilledema and visual loss in pseudotumour cerebri. Can J Neurol Sci. 1987;14:593-6.
- [Google Scholar]
- Unilateral and highly asymmetric papilledema in pseudotumor cerebri. Neurology. 1992;42(3 Pt 1):676-8.
- [Google Scholar]
- Unilateral swollen disc due to increased intracranial pressure. Neurology. 2001;56:1588-90.
- [Google Scholar]
- Preliminary data on utility of subcutaneous unfractionated heparin in patients with deep cerebral venous thrombosis. J Thromb Thrombolysis 2017 Jun 15 doi: 10.1007/s11239-017-1518-9. [Epub ahead of print] PMID: 28620756
- [Google Scholar]
- Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664-70.
- [Google Scholar]
- Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: Insights from Nizam's Institute Venous Stroke Registry, Hyderabad (India) Neurol India. 2012;60:154-9.
- [Google Scholar]
- Headache in cerebral venous sinus thrombosis revisited: Exploring the role of vascular congestion and cortical vein thrombosis. Cephalalagia 2017 DOI: 10.1177/0333102417698707 (under press)
- [Google Scholar]
- The incidence of cerebral venous thrombosis: A cross-sectional study. Stroke. 2012;43:3375-7.
- [Google Scholar]
- Cerebrovascular disease in North-West India: A study of necropsy material. J Neurol Neurosurg Psychiatry. 1989;52:512-5.
- [Google Scholar]
- Hereditary thrombophilia in cerebral venous thrombosis: A study from India. Blood Coagul Fibrinolysis. 2013;24:540-3.
- [Google Scholar]
- Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Arch Ophthalmol. 1977;95:1448-57.
- [Google Scholar]
- Foster Kennedy syndrome: Papilledema in one eye with optic atrophy in the other eye. CMAJ. 2011;183:2135.
- [Google Scholar]
- Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262-70.
- [Google Scholar]
- Unilateral papilloedema after hepatitis B vaccination in a migraine patient. A case report including forensic aspects. Acta Ophthalmol Scand. 1999;77:722-4.
- [Google Scholar]
- Unilateral optic nerve infiltration as an initial site of relapse of acute lymphoblastic leukemia in remission. Oman J Ophthalmol. 2010;3:153-4.
- [Google Scholar]
- Unilateral papilledema after trabeculectomy in a patient with intracranial hypertension. Klin Monbl Augenheilkd. 2008;225:441-2.
- [Google Scholar]
- Pseudodrusen of the optic disc. Papilledema simulating buried drusen of the optic nerve head. J Clin Neuroophthalmol. 1989;9:273-6.
- [Google Scholar]
- Bilateral transverse sinus stenosis predicts IIH without papilledema in patients with migraine. Neurology. 2006;67:419-23.
- [Google Scholar]






