Translate this page into:
A prospective study to evaluate the use of surveillance venous ultrasonography to detect incidence of deep venous thrombosis following neurosurgical excision of brain tumors
*Corresponding author: Swapnil Yeshwant Parab, Department of Anesthesiology, Critical Care and Pain, Tata Memorial Hospital, Mumbai, Maharashtra, India. swapnil.parab@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ambulkar R, Parab SY, Vignesh B, Nagargoje V, Janu A, Parikh P, et al. A prospective study to evaluate the use of surveillance venous ultrasonography to detect incidence of deep venous thrombosis following neurosurgical excision of brain tumors. J Neurosci Rural Pract 2023;14:252-7.
Abstract
Objectives:
Patients with brain tumors are prone to develop deep venous thrombosis (DVT) following neurosurgical excision of tumor. However, there is a deficiency of knowledge about the screening method, optimum frequency, and duration of the surveillance to diagnose DVT in the post-operative period. The primary objective was to find the incidence of DVT and associated risk factors. The secondary objectives were to find the optimum duration and frequency of surveillance venous ultrasonography (V-USG) in patients undergoing neurosurgery.
Materials and Methods:
Hundred consecutive adult patients undergoing neurosurgical excision of brain tumors were included after their consent, over a period of 2 years. The risk factors for DVT were assessed in all the patients preoperatively. All patients underwent surveillance duplex V-USG of the upper and lower limbs at pre-planned time intervals in the perioperative period, by experienced radiologists and anesthesiologists. The occurrence of DVT was noted using the objective criteria. The association between the perioperative variables and the incidence of DVT was assessed using univariate logistic regression analysis.
Results:
The most common prevalent risk factors were – malignancy (97%), major surgery (100%), and age >40 years (30%). Asymptomatic DVT was detected in the right femoral vein in one patient who underwent suboccipital craniotomy for high-grade medulloblastoma, on the 4th and 9th postoperative day, making the incidence of DVT 1%. The study found no association with perioperative risk factors and could not suggest the optimum duration and frequency of surveillance V-USG.
Conclusion:
A low incidence of DVT (1%) was detected in patients undergoing neurosurgeries for brain tumors. Prevalent thromboprophylaxis practices and a shorter period of post-operative surveillance could be the reasons for the low incidence of DVT.
Keywords
Brain tumors
Craniotomy
Venous ultrasonography
Deep venous thrombosis
INTRODUCTION
Cancer increases the risk of deep venous thrombosis (DVT) by 4–7 times.[1] Patients with brain tumors are at increased risk for DVT due to pre-and post-operative limb paresis, old age, prolonged ventilation, poor sensorium, and delay in post-operative ambulation. High-grade brain tumors release tissue thromboplastin to increase the risk of DVT. It is evident from the fact that venous thromboembolism (VTE) was noted in nearly one-third of patients suffering from high-grade glioma.[2] Data from “The American College of Surgeons National Surgical Quality Improvement Project” included 10,477 neurosurgical patients among whom 3.2% of patients reported VTE.[3] In India, Borde et al. found that the incidence of DVT was 12.08% in 273 patients undergoing neurosurgery.[4] Reasons for the variable incidence of DVT include variations in the presentations of DVT, use of different diagnostic modalities, variable duration of surveillance, and varying practices of thromboprophylaxis.
There are numerous methods of diagnosing DVT in the perioperative period. These include venous compression ultrasonography (V-USG), contrast venography, impedance plethysmography, magnetic resonance imaging, and assessment of coagulation parameters such as D-dimer, soluble fibrin, and thrombin-antithrombin complexes. Among these methods, V-USG carries the highest sensitivity and specificity.[5] In addition, it is non-invasive, safe, and widely available. The asymptomatic nature of DVT mandates serial surveillance using V-USG for detecting VTE in the post-operative period. However, there is a deficiency of knowledge about the optimum frequency and duration of surveillance ultrasonography (USG). Hence, we conducted a single-centered and interventional study with the primary objective to find out the incidence of DVT in patients undergoing neurosurgical excision of brain tumors. The secondary objectives were to find out the optimal duration and frequency of surveillance V-USG to detect DVT following elective neurosurgeries.
MATERIALS AND METHODS
We conducted an interventional study at a tertiary care cancer center from August 2018 to September 2020. Approval was sought from the Institutional Ethical Committee. (IEC/900260). The trial was registered with the Clinical Trials Registry – India (CTRI) on July 12, 2018. (CTRI/2018/07/014917). We included all consecutive adult patients (>18 years) undergoing elective craniotomies for brain tumors from August 2018 to September 2020. Those, who refused to participate, and those with pre-existing chronic VTE (detected by history/examination/any previous diagnostic study), were excluded from the study. Informed consent was obtained from all patients, a day before the planned surgery.
A thorough history-taking and examination were conducted a day before surgery, in the pre-operative ward by the study team member. The presence of risk factors for DVT (categorized as “strong”, “moderate,” and “weak” as per the classification by Anderson and Spencer) was noted.[6] In addition, a venous duplex USG examination (compression USG plus Doppler waveform analysis) of upper and lower limbs was conducted in the pre-operative ward by a qualified radiologist, a day before surgery.
All the patients underwent neurosurgery as per the standard protocol of the institute. Mechanical thromboprophylaxis in the form of elastic stockings was offered to all the patients from the time of pre-operative admission to the postoperative discharge, unless contraindicated. Aggressive postoperative ambulation was pursued from the 1st post-operative day, whenever possible. Patients did not receive prophylactic pharmacological anticoagulation (administration of subcutaneous doses of unfractionated heparin 100 U/kg twice daily or low-molecular-weight heparin in doses of 0.1 mg/kg body weight once daily) unless deemed necessary by the neurosurgeon. Neuroimaging was performed on the 1st post-operative day for all the patients.
The timeline for the subsequent V-USG examinations in the post-operative period is shown in [Table 1]. Accordingly, all the patients underwent a duplex USG examination at least 4 times in the perioperative period. All V-USG examinations were performed using a high-frequency linear transducer probe with images in the B mode and color Doppler mode on the Sonosite USG machine (© Fujifilm Sonosite Inc., USA). Both lower limbs (femoral, popliteal, anterior tibial, and posterior tibial veins) and upper limbs (subclavian, axillary, and brachial veins) were assessed to detect the presence of DVT. The objective criteria for diagnosing DVT on V-USG examination, were as follows –
A filling defect in the vein suggestive of a thrombus
Venous dilatation indicative of acute thrombus
A decrease in venous diameter indicative of chronic thrombus
Absent flow indicative of a completely occlusive thrombus
Increased flow through the superficial veins
Lack of augmentation in venous flow in femoral and popliteal veins on calf squeeze (in case of lower limb thrombus).[7]
| Serial numbers of USG examinations | When was the USG examination performed? | Where was the USG examination performed? | Who performed the USG examination? | Who reviewed and confirmed the finding of the USG examination |
|---|---|---|---|---|
| First USG examination | A day before surgery | Pre-surgical ward | A qualified radiologist | Consultant radiologist |
| Second USG examination | Post-operatively before discharge from the ICU or within 48 h of surgery, whichever was possible earlier. | ICU or post-operative ward | A qualified radiologist or a qualified anesthesiologist trained in vascular ultrasonography | Consultant radiologist |
| Third USG examination | Before the discharge from the hospital or after 7 days from the second examination, whichever was earlier | Post-operative ward | A qualified radiologist | Consultant radiologist |
| Fourth USG examination | At the time of the first follow up visit or within 30 days of discharge, whichever is earlier | Radiology suit | A qualified radiologist | Consultant radiologist |
USG: Ultrasonography, ICU: Intensive care unit
All USG examinations were performed by a qualified radiologist or a qualified anesthesiologist, who was trained in performing vascular USG. Clips of the V-USG were stored in the memory of the machine. The clips were reviewed by the consultant radiologist who confirmed the findings. In case of prolonged post-operative hospital stay, V-USG was repeated on a weekly basis until the time the patient was discharged from the hospital.
In case of diagnosis of DVT (regardless of whether the patient was symptomatic or not), patients were planned to receive immediate medical or interventional management, as per the American Society of Clinical Oncology Guidelines, under the guidance of the physician.[8]
We chose a convenient sample size of 100 for the study. The demographic details of the sample population were presented in terms of frequencies and percentages. The distribution of the variables was assessed for normality using the Shapiro– Wilk test. Normally-distributed continuous variables were expressed in terms of means with standard deviations. The association between perioperative variables and the incidence of DVT was assessed using univariate logistic regression analysis. P < 0.05 indicated statistical significance.
RESULTS
[Figure 1] depicts the recruitment of the patients in the study. The demographics of the sample population are shown in [Table 2]. The most commonly operated brain tumors were astrocytic tumors (n = 48) followed by oligodendroglial (n = 10) and meningothelial tumors (n = 10) [Figure 2].
| Parameter | Values |
|---|---|
| Age (Mean±SD) years | 44.19±12.1 |
| Sex-Male: Female | 55:45 |
| ASA- PS - 1:2:3 | 58:39:3 |
| BMI (Mean±SD) (kg/square meter) | 23.9±4.2 |
| Duration of surgery (Mean±SD) (minutes) | 276±107.7 |
| Blood loss during surgery (Mean±SD) (mL) | 608±702.5 |
| Post-operative hospital stay (Mean±SD) (days) | 7.0±4.1 |
| Pre-operative motor deficit-Yes: No | 21:79 |
| Median post-operative day of 2nd USG examination | 1 |
| Median (Range) post-operative day of 3rd USG examination | 4 (1–14) |
| Median (Range) post-operative day of 4th USG examination | 12 (8–39) |
ASA PS: American society of anesthesiologists physical status, BMI: Body mass index, USG: Ultrasonography

- Recruitment of patients in the study.
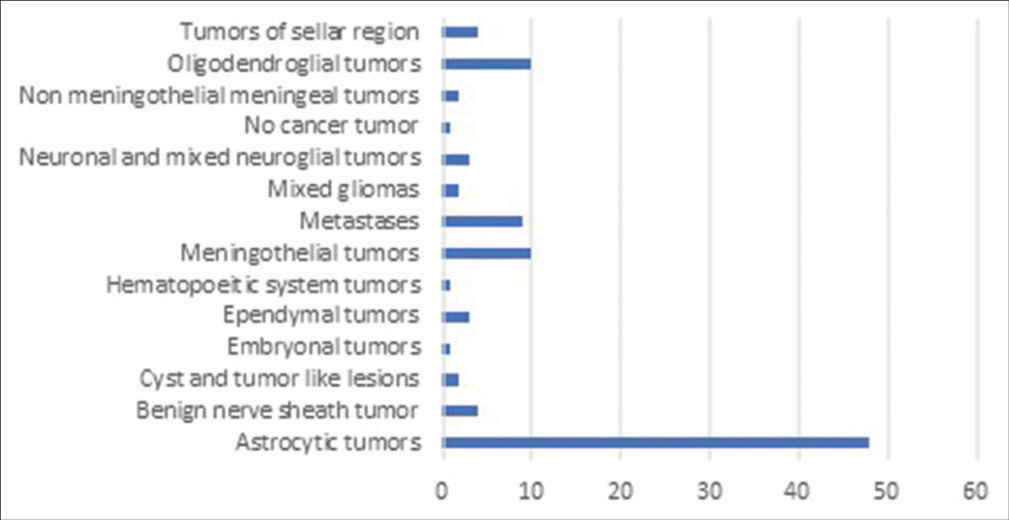
- Histopathological classification of brain tumors.
A DVT was detected in one patient during the third USG examination. The patient was a 50-year-old, American Society of Anesthesiologists (ASA) physical status-1, female who underwent the excision of high-grade medulloblastoma, by suboccipital craniotomy. The patient had no pre-operative risk factors. The intraoperative and immediate post-operative course was uneventful. Pre-discharge USG examination on the 4th post-operative day detected a partially occluding thrombus in the right femoral vein. After the diagnosis of DVT, an injection enoxaparin 0.1 mg/kg was administered twice a day for 5 days, as per the advice of the treating physician. The patient underwent another USG examination on the 9th post-operative day which showed the persistence of the thrombus. The patient was discharged with the continuation of injection enoxaparin (0.1 mg/kg once a day dose for 40 days). The follow-up USG examination on postoperative day 29 showed the complete resolution of DVT.
The prevalence of risk factors and their association with the incidence of DVT is shown in [Table 3]. The most commonly prevalent risk factors were malignancy (97%) and a history of major general surgery (100%), as neurosurgery was considered a risk factor. None of the perioperative risk factors was found to be associated with the incidence of DVT.
| Risk factors | Number of patients with the risk factor being present (%) | Incidence of DVT | Significance of the association (P-value) |
|---|---|---|---|
| Strong risk factors | |||
| Fracture of the hip/leg | 0 | 0 | |
| History of hip/knee replacement | 0 | 0 | |
| History of major general surgery | 100 (100%) | 1 (1%) | 1 |
| History of major trauma | 1 (1%) | 0 | |
| History of spinal cord injury | 1 (1%) | 0 | |
| Established coagulation disorders | 0 | 0 | |
| Moderate risk factors | |||
| History of arthroscopic knee surgery | 0 | 0 | |
| Presence of central venous lines | 1 (1%) | 0 | 1 |
| Congestive heart failure/respiratory failure | 0 | 0 | |
| Hormonal replacement therapy | 1 (1%) | 0 | 1 |
| Presence of malignancy | 97 (97%) | 1 (1.03%) | 1 |
| Oral contraceptive therapy | 4 (4%) | 0 | 1 |
| History of paralytic stroke | 6 (6%) | 0 | 1 |
| Previous history of VTE | 1 (1%) | 0 | 1 |
| Thrombophilia | 1 (1%) | 0 | 1 |
| Mild risk factors | |||
| Bed rest >3 days | 16 (16%) | 0 | 1 |
| Varicose veins | 0 | 0 | |
| Increasing age (>40 years) | 30 (30%) | 1 (3.3%) | 1 |
| Obesity (BMI >30 kg/square meters) | 3 (3%) | 0 | 1 |
| Pregnancy/postpartum | 0 | 0 |
DVT: Deep venous thrombosis, VTE: Venous thromboembolism, BMI: Body mass index
Symptomatic DVT or embolic complication was not noted in any of the patients. The average hospital stay for neurosurgery was 7.0 ± 4.1 days (median – 6 days, range – 1–27 days). Hospital mortality during the study was 1%.
DISCUSSION
The most important finding of this study was that the incidence of DVT was very low in patients undergoing neurosurgery. No association was found between the presence of risk factors and the incidence of DVT. As a result, we cannot propose the optimum timing, duration, and frequency of perioperative V-USG examination for the detection of DVT following craniotomies for brain tumors.
The incidence of DVT in patients undergoing neurosurgical excision of brain tumors varies across the world. A retrospective review of 1703 patients undergoing neurosurgery showed that the incidence of VTE was 1.59%, which was similar to the incidence found in our study.[9] A retrospective study on 9489 patients with malignant glioma found the incidence of VTE to be 7.5% in the post-operative period. Out of these, 16.1% of patients (115/715) suffered from VTE during the first 6 months and 55% of patients (399/715) were diagnosed within 61 days following the neurosurgery.[10] In another large study of 2638 patients, the majority of DVTs were noted within the first 7 days after the neurosurgery.[11] Rinaldo et al. found that the incidence of VTE was 3% and the mean post-operative day for the diagnosis of VTE was the 13th day following the surgery.[12] Hoefnagel et al. found the incidence of VTE to be 7.2% in 589 patients undergoing meningioma excision and the average post-operative day for the appearance of VTE was 21.1.[13] The inferences of these studies show that variability exists not only in the incidence of DVT but also in the time period when DVT was diagnosed. Most of the DVTs in these studies were symptomatic in nature and there was not any surveillance screening method used during the post-operative period. The reasons for the variations in the incidence of DVT could be the heterogeneous populations, different methods used for thromboprophylaxis, and different durations of follow-up after neurosurgery. It is also worth noting that the occurrence of DVT was common in the early post-operative period, but the duration of the vulnerable period extended from a few days to weeks following neurosurgery. In our study, a median day for the fourth surveillance USG was the 14th post-operative day (range from 8 to 39th post-operative day). Thus, the lack of long follow-up could be the reason for the low incidence of DVT in our study. A longer duration of surveillance USG examination, preferably for 2 months after the neurosurgery, would help cover the vulnerable period in a better way.
Different methods have different efficacies in the detection of DVT. The most commonly used method – V-USG, itself, has further variants such as duplex ultrasound and triplex ultrasound examination. Duplex ultrasound examination is a combination of color Doppler and two-dimensional venous compression examination, whereas triplex USG incorporates continuous wave Doppler in addition. In the systematic review, the accuracy of USG for the diagnosis of DVT varied according to the technique of USG and the site of vasculature screened. The sensitivity of duplex USG examination was 96% for the diagnosis of DVT in proximal veins, whereas the sensitivity for distal veins was 71%. Similarly, triplex USG (sensitivity for DVT in proximal veins – 96% and for distal veins – 75%) and compression USG alone (sensitivity for DVT in proximal veins – 94% and for distal veins – 57%) have varying diagnostic efficacies.[14] Kawaguchi et al. combined two techniques – measurement of D-dimer and Doppler USG and found a higher incidence of DVT (i.e., 14.8%) in neurosurgical patients.[15] In our study, the use of triplex ultrasound examination, measurement of D-dimer, and/or contrast venography could have improved the sensitivity of diagnosis of DVT, especially in distal veins.
In neurosurgical patients, a few risk factors are strongly associated with the occurrence of DVT. These include – old age (Hazard ratio [HR] – 2.6, confidence interval [CI] 2.0–3.4), comorbidities (HR – 3.5, CI: 2.8–4.3), histology of glioblastoma multiforme (HR – 1.7, CI: 1.4–2.1) and neurosurgery within 2 months (HR - 1.7, CI: 1.3–2.3).[9] Li et al. found that hypertension (Odd’s ratio [OR] – 3.2, 95% CI: 1.46–6.82; P = 0.003) and high post-operative D-dimer (OR - 1.2, 95% CI: 1.02–1.48; P = 0.034) were the independent risk factors for the occurrence of DVT following neurosurgery.[16] In our study, the sample population was relatively younger (mean age – 44 years), without major comorbidities (ASA 1–58%), and with no major motor weakness (power in each of the limbs ≥4 was noted in 90% of patients). These could be the reasons for the low incidence of DVT noted in our study.
There has been increased awareness about DVT prophylaxis after the evolution of “Enhanced Recovery After Surgery” (ERAS) protocols in various types of surgeries. ERAS protocols following elective craniotomies suggest limited use of intraoperative Mannitol, early extubation, and early ambulation in the post-operative period to prevent DVT.[17] In our study, compression elastic stockings were used for all the patients in the perioperative period. Furthermore, the patients with no sensory-motor deficits were made to ambulate as early as possible. Passive physiotherapy was provided to all the patients who were not able to ambulate. We think that these preventive practices would have resulted in a low incidence of DVT in our study.
A few limitations of our study must be acknowledged. First, the sample size is too less to conduct any multivariate analysis. Second, inter-operator variability could be one limitation of this study, as an anesthesiologist, intensivist, and radiologists were involved at different phases of the study. Third, the lack of long-term follow-up with surveillance USG is another limitation of the study. Being a single-centered study, prevalent thromboprophylaxis practices at our institute could have resulted in a low incidence of DVT. A multicentered study, where thromboprophylaxis practices vary, could be ideal to detect the incidence of DVT. Furthermore, we used a single method for surveillance. A combination of different methods (e.g., duplex ultrasound with D-dimer or ultrasound with contrast venography) could have improved the diagnostic accuracy of the screening technique. Finally, in our study, we had a heterogeneous mix of brain tumors with many being low-grade cancers. A more focused approach to high-grade brain tumors would have resulted in a higher incidence of DVT. In spite of all the limitations, in our study, we could include 100 patients who were repeatedly examined for the occurrence of DVT. The presence of risk factors for DVT was noted in all the patients, along with their neurological deficits. Thus, the study generates useful, although small, data regarding the use of surveillance venous USG for the detection of DVTs in patients undergoing neurosurgical resection of brain tumors.
CONCLUSION
This study detects a low incidence of DVT in patients undergoing neurosurgical resection of brain tumors, especially in the early post-operative period. Due to the low incidence of DVT, the study finds no significant association with any of the risk factors for DVT. We suggest a larger sample size, the use of combined methods of DVT screening, and longer post-operative surveillance to detect the incidence of DVT following neurosurgery for brain tumors.
Declaration of patient consent
Ethical clearance was obtained with the trial registered with the Clinical Trials Registry – India (CTRI) on 12th July 12, 2018. (CTRI/2018/07/014917) and Institutional Ethical Committee approval number is IEC/900260.
Conflicts of interest
The study received intramural funds from the “Hospital Research Administrative Council”.
Financial support and sponsorship
Nil.
References
- Management of venous thromboembolism in patients with cancer. J Thromb Haemost. 2011;9:316-24.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombosis in brain tumors. Semin Thromb Haemost. 2014;40:325-31.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of venous thromboembolism risk in patients undergoing craniotomy. World Neurosurg. 2015;84:1372-9.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of deep venous thrombosis in patients undergoing elective neurosurgery-a prospective cohort-based study. Neurol India. 2017;65:787-93.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of deep vein thrombosis of the lower extremity: A systematic review and meta-analysis of test accuracy. Blood Adv. 2020;4:1250-64.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-6.
- [CrossRef] [Google Scholar]
- Criteria for ultrasound diagnosis of deep venous thrombosis of lower extremities. J Med Ultrason. 2008;35:33-6.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189-204.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolism after brain tumor surgery: A retrospective review. Neurosurgery. 1991;28:859-63.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106:601-8.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolism: Deep venous thrombosis and pulmonary embolism in a neurosurgical population. J Neurosurg. 2011;114:40-6.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolic events in patients undergoing craniotomy for tumor resection: Incidence, predictors, and review of literature. J Neurosurg. 2019;132:10-21.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg. 2014;123:150-4.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6.
- [CrossRef] [PubMed] [Google Scholar]
- Early detection of venous thromboembolism in patients with neuroepithelial tumor: Efficacy of screening with serum D-dimer measurements and Doppler ultrasonography. J Neurooncol. 2011;101:495-504.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical predictive factors of lower extremity deep vein thrombosis in relative high-risk patients after neurosurgery: A retrospective study. Dis Markers. 2020;2020:5820749.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced Recovery After Surgery strategies for elective craniotomy: A systematic review. J Neurosurg. 2021;135:1857-81.
- [CrossRef] [PubMed] [Google Scholar]







