Translate this page into:
Stereotactic biopsy for brain lesions: Doing more with less
*Corresponding author: Ved Prakash Maurya, Department of Neurosurgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. vpmsurgery@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh M, Ahamed TP, Maurya VP, Gupta P, Bhaisora KS, Srivastava AK, et al. Stereotactic biopsy for brain lesions: Doing more with less. J Neurosci Rural Pract. 2024;15:95-102. doi: 10.25259/JNRP_258_2023
Abstract
Objectives:
Stereotactic biopsy (STB) is a potential diagnostic tool considering its minimal invasiveness, high diagnostic yield, and minimal associated complications. Over the years, various frame-based instrument systems and frameless stereotactic biopsy systems have emerged to be employed in clinical use. With this study, we intend to get more by doing less in the form of STB for the patients of doubtful intracranial lesions treated over the past 5 years. We also want to highlight the technique of performing the procedure under scalp block, which can be used as a versatile tool in many clinical scenarios. Stereotactic biopsies may be planned even in rural district-level health facilities. One-time investment to procure instruments and avail existing imaging can lead to establishing definitive diagnoses in many doubtful cases. This will result in lesser cost and early establishment of treatment. Independent risk factors determining the outcome, such as deep-seated lesions, associated edema, and intraoperative hypertension, were studied. Establishing the diagnosis helped in prognosticating the disease, explaining the natural progression of symptoms, and starting adjuvant therapy. This tissue biopsy would also help secure samples for research and molecular analysis.
Materials and Methods:
Twenty patients underwent STBs at our institution between January 2018 and December 2022. We retrospectively analyzed patient characteristics, tumor pathology, surgical procedures, and outcomes, including the diagnostic value and surgery-related complications. These patients were followed up, and their progression-free and overall survival were analyzed. The need for adjuvant treatment was noted and analyzed. All procedures were performed using Cosman Roberts Wells® stereotactic frame. Pre-procedure magnetic resonance scans were performed at the time of admission. Contrast-enhanced computerized tomography (CT) scan after frame application was performed to identify targets and calculate the coordinates. A post-procedure CT scan was done to confirm the accessibility of the targeted lesion.
Results:
The most common location of the tumor was a deep-seated thalamic lesion. A definitive diagnosis was established in 19 patients (95%) at the first STB. The diagnoses were glioma in 55% of cases, primary central nervous system lymphoma, tuberculosis, and demyelinating disorders in 10% of each, and a metastatic brain tumor in 1 (5%). The post-operative complications were all transient except in one patient with deterioration of motor weakness. The follow-up was noted, and modes of adjuvant treatment needed in these patients were recorded.
Conclusion:
Stereotactic biopsy is a useful and effective method for achieving a definitive diagnosis and aiding in treating multifocal or small deep-seated lesions in or around eloquent regions.
Keywords
Stereotactic biopsy
Scalp block
High-grade glioma
Lymphoma
Local anesthesia
INTRODUCTION
Based on the spatial coordinates detected in radiology, stereotactic biopsy (STB) is a tissue biopsy of any intracranial lesion through a small burr hole. This mode of attaining tissue biopsy is employed when the lesion is not ideal for open surgical resection, possibly due to its smaller size, depth, multifocality, or diffuse nature. Stereotactic biopsy is used in clinical conditions with lesions situated in the eloquent cortex or when a pathological diagnosis is mandatory for further management.[1] Stereotactic biopsy was first conceived by Clarke and Horsley, who, in the early 1900s developed the primary device for targeting cerebellar structures in animals.[2,3] Since its invention, the technique has imbibed and adapted its fundamentals using the latest advances. At present, it is the least invasive procedure to obtain a sample for pathological diagnosis and evaluation for therapeutics in patients with intracranial lesions. The procedure can be carried out with a frameless or a frame-based stereotactic method, neuro navigation, or robotic assistance in surgery. Recent studies have failed to find statistically significant differences between frameless and the frame-based biopsy methods in the diagnostic yield, mortality, morbid complications, and after-procedure complications.[4] Commonly employed frame-based systems are the Cosman Roberts Wells (CRW) (Integra et al. Cincinnati, USA) and Leksell® (Elekta et al.) stereotactic systems. Frameless or stereotactic navigation systems employ several small fiducial markers fixed over areas of the scalp, which act as reference landmarks.
Despite the wide utilization of stereotactic biopsy, the results found in various studies on STB are controversial and non-comparable.[5] Our department has performed frame-based brain biopsies since 2018. Here, we share our experiences of stereotactic biopsy performed with the frame-based system under local anesthesia over the past 5 years. Besides focusing on the clinical spectrum and diagnostic yield, the study also highlights the essential anesthetic and surgical techniques stepwise to fine-tune the routine practice in clinics of stereotactic biopsy.
MATERIALS AND METHODS
All the patients who had undergone a frame-based stereotactic biopsy between January 2018 and December 2022 were eligible for inclusion. The data were retrieved from the hospital information system. The collected parameters were the age of the patient, sex, provisional/working diagnosis, and comorbidities. Pre-operative radiological diagnosis, the anatomic region involved in the lesion, and the Karnofsky’s performance status (KPS) score were noted. Since the procedures were performed under scalp block, the patient’s cooperation undergoing the frame-based STB was crucial. The number of samples taken from the lesion, duration of hospital stays, or any complications following the procedure was recorded. Intraoperative impression and histopathology provided a base for further line of treatment.
All procedures were performed using a CRW® stereotactic frame. Pre-operative magnetic resonance imaging (MRI) scans under neuro navigation protocol were performed at admission. After the frame was applied, a contrast-enhanced computerized axial tomography (CAT) scan was utilized to target the lesion and to calculate Cartesian coordinates. Using these measurements on three axes (X, Y, and Z), the targeted lesion was biopsied under an aseptic condition. Routine post-operative care was provided as per the departmental protocol. Post-procedure CAT scan was done to look for the biopsied target lesion.
Stereotactic frame
The CRW stereotactic-based frame is a universal compact head ring assembly. The significant parts of this assembly include a universal head ring, adapter plates, curved and short head posts, crossbars for pediatric patients, pointers, tubes, localizer frames, screws, pins, and head ring wrenches. [Figure 1a-d] The attachments of the CRW frame are fixed on the patient’s head and are carried out by drawing a canthomeatal line, and the frame is kept parallel to it, which acts as a reference line. This canthomeatal line corresponds to the radiological line drawn from anterior to posterior commissure in the midline.
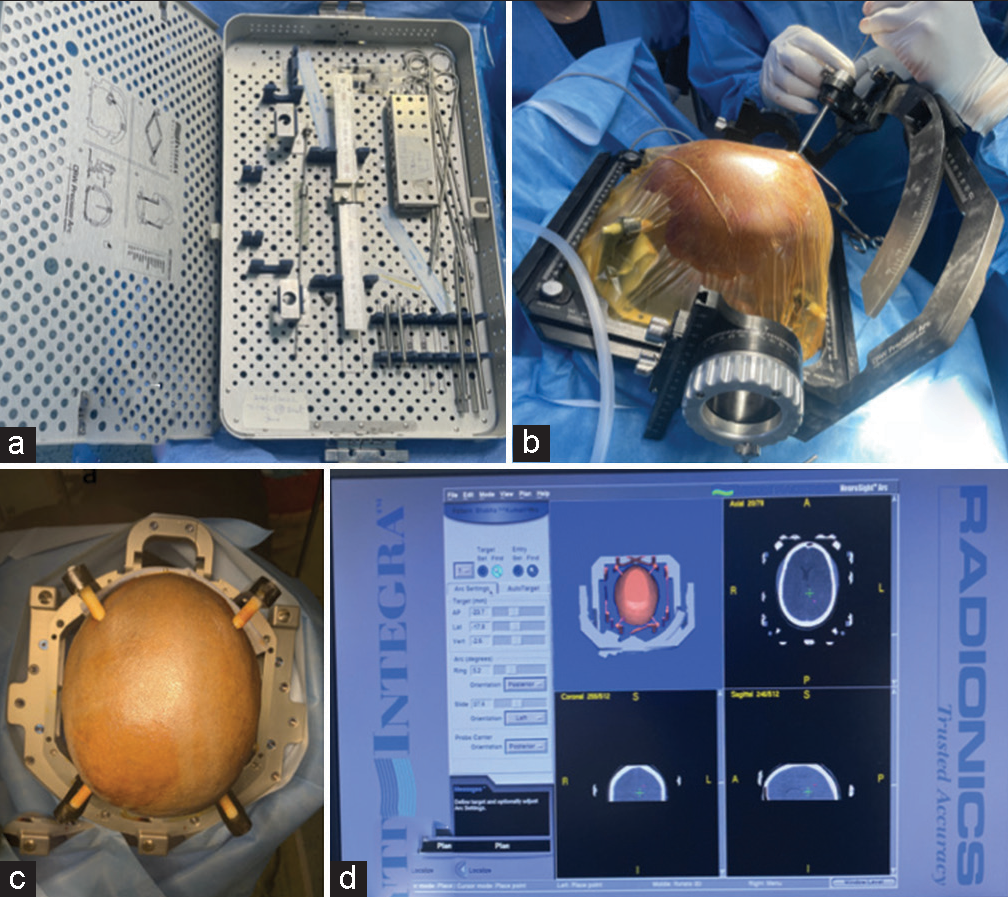
- (a) Cosman-Roberts-Wells stereotactic biopsy set, (b) inserting biopsy needle, (c) pins placed in orbitomeatal line, (d) planning in work station.
Scalp block
Scalp block is the selective blockade of specific scalp nerves using local anesthesia. The benefits of scalp block can be utilized for different sub-types of awake cranial clinical procedures such as stereotactic-based biopsy, surgery for deep brain stimulation, and stereotactic radiosurgery. Relevant history and physical examination including airway examination and evaluation regarding suitability for awake procedure in terms of cooperation were performed.[6]
The nerves that are blocked for scalp block were chosen according to the incision and craniotomy planned. For a complete scalp anesthesia, six nerves are blocked on both sides using about 40 mL of local anesthetic [Figure 2a-d]. Long-acting local anesthetic are administered along with epinephrine in concentration 1:200,000 to prolong the duration of action and reduce systemic absorption. Bupivacaine 0.5% is the most used local anesthetic agent. The maximum dose limit of local anesthetic is calculated according to body weight [Table 1, upper half].

- (a and b) Showing front and side views, respectively, of cranial nerves to be blocked. A-Supratrochlear nerve, B-Supraorbital nerve, C-Zygomaticotemporal nerve, D-Auriculotemporal nerve, E-Lesser occipital nerve, F-Greater occipital nerve, G-Great auricular nerve. (c and d) Showing scalp and forehead sensory innervation with dermatome-wise distribution. V1: Ophthalmic nerve, V2: Maxillary nerve, V3: Mandibular nerve.
| Local anesthetic | Maximum safe dose | Onset of action | Duration of action |
|---|---|---|---|
| Lidocaine 2% | 4 mg/kg | 60–90 s | 20–30 min |
| Lidocaine with epinephrine | 7 mg/kg | 60–90 s | Up to 2 h |
| Bupivacaine 0.5% | 2 mg/kg | 10–20 min | 4–6 h |
| Bupivacaine with epinephrine 0.5% | 3 mg/kg | 10–20 min | Up to 8 h |
| Nerve to be blocked | Origin | Anterior craniotomy | Posterior craniotomy |
| Supratrochlear | Ophthalmic branch of trigeminal V1 | Blocked | Not blocked |
| Supraorbital | Ophthalmic branch of trigeminal V1 | Blocked | Not blocked |
| Auriculotemporal | Mandibular branch of trigeminal V2 | Blocked | Not blocked |
| Zygomaticotemporal | Maxillary branch of trigeminal V2 | Blocked | Blocked |
| Greater occipital | C2 posterior ramus | Not blocked | Blocked |
| Lesser occipital | C2 and C3 ventral rami | Not blocked | Blocked |
For sedation, before application of the block, initially a loading dose of 1 mg/kg dexmedetomidine is given intra venous in 20 min, followed by infusing continuously a maintenance dose of 0.1–0.7 mg/kg/h. Scalp block is given under aseptic conditions. 3–5 mL of local anesthetic is infiltrated using a 24-gauge needle for each branch which supplies sensory function of the forehead and scalp [Table 1, lower half].
Supraorbital nerve (N)
The finger palpates the supraorbital notch. The needle is inserted at a site half an inch medial to the supraorbital notch along the upper edge of the orbit.
Supratrochlear N
It exits superomedially at an angle of the orbit and traverses parallel to the supraorbital nerve one-inch medial to the forehead. It is anesthetized by extending the supraorbital block to the medial side.
Zygomaticotemporal N
Infiltration was done from the supraorbital ridge to the posterior part of the zygomatic arch. Deep and superficial injections are given.
Auriculotemporal N
It was blocked with infiltration of the zygomatic protrusion at the level of the tragus on 1–5 cm anterior side. The superficial temporal artery should be palpated before the block as it lies at the level of the tragus.
Greater occipital N
Infiltration was done 2.5 cm away from the nuchal line laterally in the middle of the mastoid and occipital protuberance. The occipital artery is palpated, and an injection was given medially.
Lesser occipital N
Block is given 2.5 cm lateral to the greater occipital nerve along the nuchal line.
Stereotactic biopsy procedure
Pre-operative MRI was done before admission, and desired sequences were obtained in a compact disc. The fixation of the frame is done on the same day of the biopsy, after which a contrast-enhanced CAT scan is performed with the frame in situ. Images of MRI and computerized tomography (CT) scans are fused, and the biopsy is planned in the workstation. Usually, the enhancing part away from vital structures and avoiding the necrotic part was targeted. The entry point was usually selected on the non-dominant pre-coronal area. A suitable trajectory was identified, keeping in mind to avoid ventricles, blood vessels, and sulci. The coordinates in the X, Y, and Z coordinates, ring, and arc angles are obtained using a phantom in the operation room. Standard surgical cleaning and draping are done, and the coordinates are fixed on the arc. The burr hole was marked on the skull after passing the pointer through the arc of the frame. The dura was incised, margins coagulated, and the underlying pia was gently opened after coagulation, avoiding cerebrospinal fluid egress, which may result in a brain shift. The biopsy needle is calibrated so that the tip hits the target. Attempts are made to take at least 3–4 biopsy cuts from all the quadrants of the lesion. After obtaining an adequate biopsy, 0.5 mL of air is injected into the tumor cavity, which helps to identify the biopsy site in a post-operative CT scan. Two targets were chosen along a single needle tract, one from the center and the other from the periphery, to collect three to four samples in a circular pattern from each selected target.
RESULTS
Study population
During the duration of the study, 20 frame-based stereotactic brain biopsies were conducted on 20 patients with a mean age of 42 years (range 18–66 years). Males and females were equal in our study cohort. KPS ranged from 40 to 90, with most patients having a KPS of 80 (able to carry out regular activity with effort). Most patients spent 1–2 days in a postoperative neurosurgery unit before being discharged with regular post-operative follow-up.
Results from the biopsy procedures
A pathological diagnosis was final in 19 of the 20 biopsies done with a diagnostic yield of 95.0%. The histopathological diagnosis and sites of the lesion are presented in Table 2a and b, respectively. The lesion’s average size in the largest dimension was 2.43 cm (1.8–3.75 cm). An average of two biopsy samples was taken during each procedure (1–5 biopsies). The most common biopsy indication was a deep-seated thalamic tumor. The presence of an air pocket was noted in all the post-operative scans and was noted to correspond to the site of the lesion.
| a | |
| Histopathological diagnosis | Frequency |
| Grade 4 astrocytoma | 4 |
| Grade 2 astrocytoma | 4 |
| Low-grade glioma | 2 |
| Lymphoma | 2 |
| Tuberculosis | 2 |
| Demyelination | 2 |
| Grade 3 astrocytoma | 1 |
| No malignancy/granuloma | 1 |
| Metastasis | 1 |
| Inconclusive | 1 |
| b | |
| Location of tumor | Frequency (%) |
| Thalamic lesions | 8 (40) |
| Frontal lesions | 4 (20) |
| Peri trigonal | 3 (15) |
| Parieto-occipital | 2 (10) |
| Multi-focal lesion | 2 (10) |
| Corpus callosum | 1 (5) |
Complications
There was no procedure-related mortality in the study cohort. Transient neurologic deficit was noted in two patients (10%). One patient developed a post-operative hematoma, another patient had a fall in the Glasgow Coma Scale score, and CT revealed brain edema; both were managed conservatively with no additional procedure-related morbidity. A permanent neurologic deficit was noticed in a patient (5%) with a suitable thalamic space-occupying lesion who presented with the left hemi paresis (Medical Research Council grade 4/5), which further reduced to 3/5 at discharge.
In one patient, no definitive histological diagnosis could be arrived at (5%). However, with a high index of suspicion due to clinical and imaging characteristics, the patient was taken up for a definitive surgical procedure. Craniotomy and decompression of the tumor were performed, and a histopathological examination of whom turned out to be a World Health Organization (WHO) grade 4 Glioma.
Total procedure time
Operating time data and the overall procedure time were taken from the theater database. We defined the overall time for stereotactic biopsies to include the administration of scalp block anesthesia, positioning of the frame, transporting the patient to the CT and back into the operating room, and the biopsy procedure itself. The mean overall procedure time was 4 h 45 min (range 3 h 50 min–6 h and 15 min).
Anesthesia method
All stereotactic biopsies were performed under scalp block (local anesthesia), which was tolerated well, and none of these patients required secondary general anesthesia. All patients underwent a post-operative CT scan within 6 h of the procedure.
Follow-up
A total of 19 patients were followed, and the average follow-up duration ranged from 3 months to 39 months. Eight patients received adjuvant therapy in the form of chemo and radiotherapy. Following therapy, seven patients are in regular follow-up, accounting for 35%, whereas 12 patients expired between 3 and 23 months following diagnosis. One patient with metastatic carcinoma esophagus could not be traced and was lost to follow-up after 2 months.
DISCUSSION
Stereotactic needle biopsy is an established procedure for studying characters of lesions situated in the brain whose clinical picture is unclear or not found optimal for surgical management. The procedure can be done using frameless (navigation/robot-assisted) or frame based as in our study. Stereotactic needle biopsy is considered a prudent and precise approach for the management of deep-seated brain lesions and those located in eloquent areas.[7]
Frame-based stereotactic biopsy employs a rigid frame fixed firmly over the patient’s head at the beginning of the procedure. This technique is based on three-dimensional coordinates, where the target point (intracranial lesion) is given a coordinate relative to a reference point. This reference point was defined over the frame based on the pre-operative imaging sources (intracranial CT and MRI). The major limitation of the frame-based biopsy technique is the complexity and maintenance of the frame. The patient’s discomfort and prolonged procedure time are additional disadvantages of this technique.[8,9] The frameless neuronavigation system utilizes a pointer, a digitizer, and a fiducial system. The fiducials are placed on the patient’s scalp before imaging and are then used as a reference to allow for the target calibration relative to the patient’s head. Registering and transferring this spatial information allows a registered pointer and the biopsy probe to be projected onto the pre-operative images. By doing this, an accurate intraoperative navigation and targeting of the set lesion is achieved. The actual surgical duration is shortened since the imaging and planning of the target are separate from surgery concerning time and location. The major drawback of the frameless biopsy technique is because of more drift and tremor of the surgeon due to the complex hand-eye coordination as a pre-requisite for this modality.[10,11]
The main reason to choose stereotactic biopsy rather than craniotomy is that stereotactic biopsies have minimal adverse effects with a high diagnostic accuracy rate. Stereotactic biopsy has advantages over open biopsy in tumors with characteristics like:
Lesions that do not result in mass effect or are not amenable to excision, such as deep-seated metastatic deposits or intrinsic brain tumors of doubtful nature;
Deeply located lesions or those found in eloquent regions or deep nuclei (e.g., basal ganglia and thalamus), for which open surgical procedure would result in unacceptably complications and
Infiltrative diseases (e.g., gliomatosis cerebri) that lack a clear brain-tumor interface and are less likely to be excised without significant loss of unaffected neural tissue.
Furthermore, if the lesion, on imaging or the disease progression, suggests an alternative pathology, such as an infectious or demyelinating disease, stereotactic biopsy is a well-considered first step rather than a more invasive procedure like craniotomy.
Tumors with high vascularity such as renal cell carcinoma, choriocarcinoma, or metastatic melanoma are relative contraindications. They are generally not approached with STB because of the high risk for hemorrhage, deranged coagulation parameters, bad general conditions, irritability, and noncompliance to cooperate during the procedure. In cases of metastatic tumors, if the patient’s condition is stable, primary underlying pathology should be looked for and treated it accordingly. Besides, tumors close to a major blood vessel, the vessel-rich Sylvian fissure, the cavernous sinus, should make the neurosurgeon consider the risk for hemorrhage. When possible, stereotactic biopsy should also be avoided in patients undergoing treatment with anticoagulant drugs.
The diagnostic correctness in our study of the frame-stereotactic biopsy technique was 95.0%. The accurate tissue yield in the frame-stereotactic technique was 84.21– 97.5%.[12-15] In a study done by Jain et al. among 95 patients,[12] accurate diagnosis was arrived at in 80 patients (84.21%), whereas Dorward et al. achieved a higher diagnostic yield of around 95% among 75 cases of frame-based biopsy.[16] High-grade glioma was the most common tissue diagnosis, accounting for 40% of all cases. In the research conducted by Tsermoulas et al., in a total of 124 individuals, diagnostic accuracy was 93.5%, with Glioblastoma (GBM) being the most frequent finding (41.1%), followed by B-cell lymphoma (17.74%), which is similar to our study.[14]
Historically, small biopsies yielded inadequate viable tissue for obtaining the essential molecular diagnosis; however, technological advancement resulted in even smaller specimen samples being analyzed by an array of molecular markers. Only with the availability of advanced pathological techniques and know-how, these biopsies are adequate to gain all necessary information for accurate impression in case open resection is not deemed necessary. As mentioned, 95% of our biopsies shed light on microscopic histology and molecular uniqueness of the tumor. A precondition for a correct molecular diagnosis is to acquire the sample out of the solid regions of the tumor. The neuropathologist must be well versed in working on small samples.
Surgical management of brain tumors is guided by three basic principles: Oncologic clearance, preserving and restoring function, and improvement in quality of life.[17] Surgical decisions are based on achieving either one or more of these aims. GBM, WHO grade 4 is the most common malignant primary central nervous system tumor, with a poor prognosis of only 12–16 months survival despite adequate treatment consisting of maximal safe surgical resection, adjuvant or concomitant chemotherapy, and radiation therapy.[18,19] Challenges in treatment are compounded by the significant heterogeneous nature of the tumor, microscopic invasion, and difficulty in differentiating margins of the lesion from normal brain intraoperatively.[20,21] All glioma treatment depends on a tissue diagnosis, with molecular analysis supplementing histopathologic analysis. Where possible, gross total resection remains the gold standard. For lesions not amenable to gross total resection, a biopsy is recommended to achieve a convincing diagnosis if radiation and chemotherapy are considered adjuvant therapy to achieve progression-free survival.
The mean size of the tumors in our analysis was 2.43 cm, values that ranged from 1.8 to 3.75 cm. In a study by Dorward et al., the mean size of the lesion was 3.48 cm, with a range from 0.8 cm to 8 cm among the frame-based group.[16] Various literatures suggests that the brain lesion volume influences the diagnostic yield. Larger lesions are associated with more accurate biopsies and vice versa. In the present study cohort, only one among the various biopsies was inconclusive. A previous study had an overall negative report in 15.79% of patients.[12] Since a high index of suspicion was kept, the patient underwent a craniotomy and excision of the tumor, which was reported as a WHO Grade 4 GBM.
Neuro-oncology is changing rapidly; classification and treatment are based on indispensable immune-histochemistry and molecular biological data, highlighting the role of a biopsy in treating intracranial tumors.[22] In 2012, Jakola et al. and Sanai and Berger documented that safe maximum tumor resection, made possible by the correct use of frameless image-guided surgery, is associated with better prolonged overall survival of both low-grade and high-grade gliomas.[20,23] Meanwhile, the recently introduced WHO classification of brain tumors advocates for correct molecular profiling of tumors of the brain.[24]
Although STB is a minimally disruptive procedure, severe complications can occur in up to 8% of cases. Thus, obtaining adequate quantities of diagnostic tissue with a minimum number of samples is of paramount concern.[7] Bleeding is the most frequent operative complication encountered post-stereotactic biopsy, with symptomatic and asymptomatic hemorrhage incidence ranging from 1.3% to 59.8%. Kulkarni et al. divided new hemorrhages by lesion size and location and found that 41.1% of the 56 intraparenchymal hemorrhages were smaller than 5 mm in maximal length.[25] Mizobuchi remarked that hemorrhages were lesser than 5 mm in size in 22 of the total 25 patients with post-biopsy hemorrhages.[26] A high-density region < 5 mm at the site where the biopsy needle was inserted on post-operative CAT scans was not counted hemorrhagic; rather, it represents merely an postoperative change after stereotactic surgery.[27]
Deeply located lesions associated with edema and dye uptake on contrast imaging, intraoperative rise in blood pressure in a non-hypertensive patient, and malignant glioma were autonomous risk factors associated with hemorrhage after stereotactic tissue acquisition.[7,28-30] These results pointed toward the possibility of bleed occurring after biopsy increases in a higher grade of malignant tumors with abnormal vasculature. The mortality rate due to acquiring tissue stereotactically ranges from 0% to 4%,[7] and total morbidity varies from 0 to 13%.[30,31] Hence, careful optimization, correct acquisition of the desired lesion site, and intraoperative management has to be ensured while performing stereotactic needle procedures for higher-grade glial lesions, lymphomas, and lesions with high vascularity.
Limitations of the study
An inherent limitation of stereotactic biopsy is that the surgical coordinates are based on information obtained from pre-operative imaging. Several factors can lead to mis-registration of stereotactic coordinates on imaging with actual physical location. Sampling error concerns stereotactic biopsies, particularly with non-enhancing lesions like low-grade gliomas. It is a time-consuming procedure accounting for time both inside and outside the operating room. We have not employed frozen section or fluorescence guidance in our study, which has been shown to increase the diagnostic yield.
CONCLUSION
The stereotactic biopsy system should be considered an essential surgical tool in the neurosurgeon’s armamentarium and must be utilized in specific patient populations. The procedure is time-consuming but has a high diagnostic yield, aids in optimizing management, and improves patient care and outcomes.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)- assisted technology in manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- How is stereotactic brain biopsy evolving? A multicentric analysis of a series of 421 cases treated in Rome over the last sixteen years. Clin Neurol Neurosurg. 2018;174:101-7.
- [CrossRef] [PubMed] [Google Scholar]
- Stereotactic neurosurgery in the United Kingdom: The hundred years from Horsley to Hariz. Neurosurgery. 2008;63:594-606.
- [CrossRef] [PubMed] [Google Scholar]
- THE CLASSIC: On a method of investigating the deep ganglia and tracts of the central nervous system (cerebellum). Br Med J 1906:1799-1800. Clin Orthop Relat Res. 2007;463:3-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of frame-based versus frameless intracranial stereotactic biopsy: Systematic review and meta-analysis. World Neurosurg. 2019;127:607-16.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Frame-based stereotactic biopsy: Description and association of anatomical, radiologic, and surgical variables with diagnostic yield in a series of 407 cases. J Neurol Surg A Cent Eur Neurosurg. 2019;80:149-61.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic and bispectral index changes following skull pin attachment with and without local anesthetic infiltration of the scalp. J Anesth. 2007;21:442-4.
- [CrossRef] [PubMed] [Google Scholar]
- The risk factors of hemorrhage in stereotactic needle biopsy for brain lesions in a large cohort: 10 years of experience in a single center. Chin Neurosurg J. 2022;8:40.
- [CrossRef] [PubMed] [Google Scholar]
- Blurring the boundaries between frame-based and frameless stereotaxy: Feasibility study for brain biopsies performed with the use of a head-mounted robot. J Neurosurg. 2015;123:737-42.
- [CrossRef] [PubMed] [Google Scholar]
- Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003;26:73-99.
- [CrossRef] [PubMed] [Google Scholar]
- Frameless stereotactic targeting devices: Technical features, targeting errors and clinical results. Int J Med Robot. 2012;8:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23:71-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic yield of stereotactic brain biopsy. Neurosciences (Riyadh). 2008;13:142-5.
- [Google Scholar]
- Factors affecting diagnostic yield in needle biopsy for brain lesions. Br J Neurosurg. 2013;27:207-11.
- [CrossRef] [PubMed] [Google Scholar]
- Computed imaging stereotaxy: Experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-7.
- [CrossRef] [PubMed] [Google Scholar]
- The advantages of frameless stereotactic biopsy over frame-based biopsy. Br J Neurosurg. 2002;16:110-8.
- [CrossRef] [PubMed] [Google Scholar]
- Updates on surgical management and advances for brain tumors. Curr Oncol Rep. 2021;23:35.
- [CrossRef] [PubMed] [Google Scholar]
- Glioblastoma: Overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2-8.
- [CrossRef] [PubMed] [Google Scholar]
- CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Suppl 5):v1-100.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical oncology for gliomas: The state of the art. Nat Rev Clin Oncol. 2018;15:112-25.
- [CrossRef] [PubMed] [Google Scholar]
- Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed Pharmacother. 2017;92:681-9.
- [CrossRef] [PubMed] [Google Scholar]
- Improving diagnosis and management of primary brain tumors. Curr Opin Neurol. 2017;30:639-42.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308:1881-8.
- [CrossRef] [PubMed] [Google Scholar]
- A single-centre study of frame-based stereotactic brain biopsies. Br J Neurosurg. 2022;36:213-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89:31-5.
- [CrossRef] [PubMed] [Google Scholar]
- The risk of hemorrhage in stereotactic biopsy for brain tumors. J Med Invest. 2019;66:314-8.
- [CrossRef] [PubMed] [Google Scholar]
- Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J Neurosurg. 2005;102:897-901.
- [CrossRef] [PubMed] [Google Scholar]
- Hemorrhage after stereotactic biopsy from intra-axial brain lesions: Incidence and avoidance. J Neurol Surg A Cent Eur Neurosurg. 2014;75:177-82.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr. 2017;20:261-8.
- [CrossRef] [PubMed] [Google Scholar]
- Complications following stereotactic needle biopsy of intracranial tumors. World Neurosurg. 2015;84:1084-9.
- [CrossRef] [PubMed] [Google Scholar]
- Frameless image-guided stereotactic brain biopsy procedure: Diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006;104:233-7.
- [CrossRef] [PubMed] [Google Scholar]






