Translate this page into:
Association between ApoE ε4 genotype and attentional function in non-demented, middle-aged, and older adults from rural India
*Corresponding author: Jonas S. Sundarakumar, Centre for Brain Research, Indian Institute of Science, Bengaluru, Karnataka, India. sjonas@iisc.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Rai P, Sundarakumar JS, Basavaraju N, Kommaddi RP, Issac TG. Association between ApoE ε4 genotype and attentional function in non-demented, middle-aged, and older adults from rural India. J Neurosci Rural Pract. 2024;15:117-25. doi: 10.25259/JNRP_272_2023
Abstract
Objectives:
Several genetic factors have been associated with cognitive decline in aging. Apolipoprotein E (ApoE) ε4 has been widely studied in the risk for pathological cognitive decline, including dementia. However, the association between ApoE ε4 and cognitive functioning in the healthy aging Indian population has been understudied, and the results are ambiguous.
Materials and Methods:
This study aims to examine the role of the ApoE genotype with attentional function in aging adults (≥45 years) in a rural Indian population. Cross-sectional (baseline) data (n = 2100) was utilized from an ongoing longitudinal cohort study on aging (Srinivaspura Aging, Neurosenescence, and Cognition study). Participants hailed from villages of Srinivaspura in Karnataka, southern India. Participants were categorized based on ApoE-ε4 status into three categories: No ε4, heterozygous ε4, and homozygous ε4. Attentional function was assessed using the auditory and visual attention subtests from a computerized neurocognitive test battery. Linear regression was performed adjusting for age, gender, and education.
Results:
In model 1 (unadjusted), we did not find an association between ApoE and attention function. In the partially adjusted model 2 (adjusting for age), ApoE ε4 with age was significantly associated with the attention function. Further, with increasing age, there was a decline in attention among homozygous ε4 individuals. Model 3 (model 2 + gender) found that ApoE ε4, age, and gender explained a significant variance in attention function. In addition, with increasing age, males had poor attention in the homozygous as compared to heterozygous group. Model 4 (model 3+ education) explained a significant variance in attention and also revealed that with increasing age, attention declined in the illiterate and low literacy groups in both homozygous and heterozygous groups among both genders.
Conclusion:
Although ApoE ε4 alone was not associated, it interacted with age, gender, and education to affect attention function in this rural Indian population. Longitudinal cognitive monitoring will yield insights into understanding whether the ApoE ε4 genotype influences the rate of cognitive decline in this rural, aging population.
Keywords
Apolipoprotein E
Cognition
Attention
Aging
Rural Indians
INTRODUCTION
Aging is associated with dynamic changes in biological, behavioral, environmental, psychological, and social processes. The effect of age on the brain is reflected in structural and functional changes, which, in turn, can impact cognitive functions. Aging is the primary risk factor for several neurogenerative diseases, such as Alzheimer’s disease (AD) and related disorders. Several genes are related to a greater risk of cognitive impairment and dementia in the aging population. Out of these, the role of apolipoprotein E (ApoE) gene has been one of the most widely studied.[1]
ApoE is a lipoprotein known to play an essential role in lipid transport and metabolism in the brain.[2] The three common alleles of the ApoE gene are ε2, ε3, and ε4, encoded by two single nucleotide polymorphisms (SNPs).[3,4] Several studies have identified the ε4 allele as a significant risk factor for late-onset AD.[3,4] Its detrimental effects are postulated to be through disruption of intracellular lipid homeostasis, which, in turn, affects Aβ aggregation and clearance.[5] Individuals heterozygous for ε4 are 2–3 times more likely to develop sporadic AD, and this risk increases to 8–12 times for those with homozygous ε4.[6]
However, this risk is not uniform across all populations. In Caucasian individuals, the ε4 genotype had a considerably higher chance of developing AD.[7] Japanese respondents with the ε4 genotype had a greater risk of developing AD than Caucasian subjects.[8] However, in African Americans and Hispanics, a weaker association between ApoE 4 and AD was seen.[9] A study from India revealed that ApoE polymorphism was not associated with cognitive or functional abnormalities in persons with dementia.[10,11]
Among cognitively healthy individuals, the association between ApoE ε4 genotype and cognition is inconclusive. A study on age-specific effects in the association of apolipoprotein ε4 gene and cognition showed that among people 50 years and older, the ApoE ε4 allele is significantly linked to worse memory function, with the effect being most prominent in Auditory Verbal Learning Test learning scores.[12] Another study assessing ApoE genotype and cognitive performance in a Caucasian population revealed that the ApoE ε4 allele had no impact in any of the studied age groups.[13] On the contrary, a cohort study on ApoE genotype and change in cognition in young, middle-aged, and older adults from Australia showed that ApoE genotypes are not associated with cognitive decline in any age group.[14] Therefore, results from prior studies appear largely inconclusive due to differences in findings or the designs of assessments used.
It is also essential to understand that the findings from the above studies on the association between ApoE and cognitive function among cognitively healthy individuals may not be pertinent to the Indian population due to the ethnicity-specific effects of ApoE on cognitive performance. In addition, cardiovascular risk factors that potentially mediate the effects of ApoE on cognition vary considerably between populations.[15]
Further, many of the prior studies have assessed overall cognitive performance using brief global cognitive tools like the Mini-Mental Status Examination (MMSE), which failed to provide a consistent picture of the impact of ApoE on cognition. For example, in a Brazilian cohort study among aging participants, a negative correlation was observed between the ApoE ε4 allele and scores on the MMSE’s domains of memory. However, there was no influence on the other dimensions of MMSE.[16] Therefore, it is likely that the association between ApoE ε4 and cognitive performance could be cognitive domain-specific.
Among the various cognitive domains affected in dementia, it has been shown that the attention domain is usually affected the earliest. Several neurointerventions in early dementia also target improvement in the attentional domain.[17] Hence, it becomes crucial to study the association of the ApoE ε4 genotype with attentional function among cognitively healthy individuals even before they develop dementia. However, there is a paucity of such studies worldwide, especially in the Indian population among middle-aged and older individuals with no apparent cognitive deficits. The existing Indian studies predominantly focus on urban populations, whereas studies in rural populations with low literacy levels are scarce.[18]
In the above backdrop, we aim to examine the influence of ApoE ε4 on attentional functioning among adults above 45 years of age in a rural, south Indian population. These individuals are part of an ongoing longitudinal cohort study, namely, the Srinivaspura Aging, Neurosenescence, and Cognition (SANSCOG) study,[19] which is primarily aimed at identifying risk and protective factors associated with changes in cognitive functions in normal aging, dementia, and other related disorders. We hypothesize that ApoE ε4 is associated with poorer performance in attentional function.
MATERIALS AND METHODS
Study design
The present study utilized cross-sectional (baseline) data extracted from the SANSCOG cohort study. The study protocol of the SANSCOG study has been published elsewhere.[20]
Participants and recruitment
Participants (n = 2100) were aged 45 years and above and recruited using an area sampling strategy, from a community setting in the villages Srinivaspura in Kolar district of Karnataka state in India.[19] Individuals diagnosed with dementia or severe medical/psychiatric illness and those with severe hearing/vision impairment that could impede clinical and cognitive assessments were excluded from the study.
Study assessments
Sociodemographic details
Age
The study population was stratified into three age groups: 45–54 years, 55–64 years, and 65 years and above.
Gender
The study sample was divided into two categories: male and female.
Education
Depending on their educational levels, participants were classified into three groups: literate, low literate, and illiterate.
ApoE ε4 gene status determination
Genomic DNA from whole blood was isolated using a genomic DNA isolation kit according to the manufacturer’s instructions (MACHEREY-NAGEL, Germany). Genomic DNA was diluted, and a polymerase chain reaction (PCR) was performed. The thermal conditions for PCR were programmed. The amplified PCR products were resolved on 2% agarose gel electrophoresis; the gel was excised, and its size was minimized. It was, then, extracted using QIAquick Gel Extraction Kit. The amplified PCR products were eluted in TE buffer and sequenced by the Sanger sequencing method (Applied Biosystems). After DNA sequencing, we analyzed the electropherograms of the two SNPs located within exon 4 of the ApoE gene, rs429358, and rs7412, and categorized the alleles for ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, and ε4/ε4, respectively. Participants were categorized based on the number of ε4 alleles (0, 1, or 2) into three groups: No ε4 (ε2/ε2, ε3/ε3, and ε2/ε3), heterozygous ε4 (ε2/ε4 and ε3/ε4), and homozygous ε4 (ε4/ε4).
Attentional function testing
The auditory and visual attentional tasks from a computerized, validated neurocognitive test battery, COGNITO (computerized assessment of adult information processing), were used to assess attentional function.[21] Since COGNITO is touchscreen-based and had earlier been specifically adapted to the Indian sociocultural milieu and local language, this made it feasible for our rural participants with low literacy levels.[22]
In the auditory attention task, the subject was asked to distinguish between long and short sounds provided at varying intervals [Figure 1]. In the visual attention task, the subject was shown a geometric shape for 2 seconds, followed by a target screen with two identical variations of the same shape. Then, the two similar shapes must be located visually and touched by the subject [Figure 2]. The total attention score was calculated by adding the scores from the visual and auditory attention tasks.

- This figure depicts the schematic representation of the auditory attention task used in our neurocognitive battery.
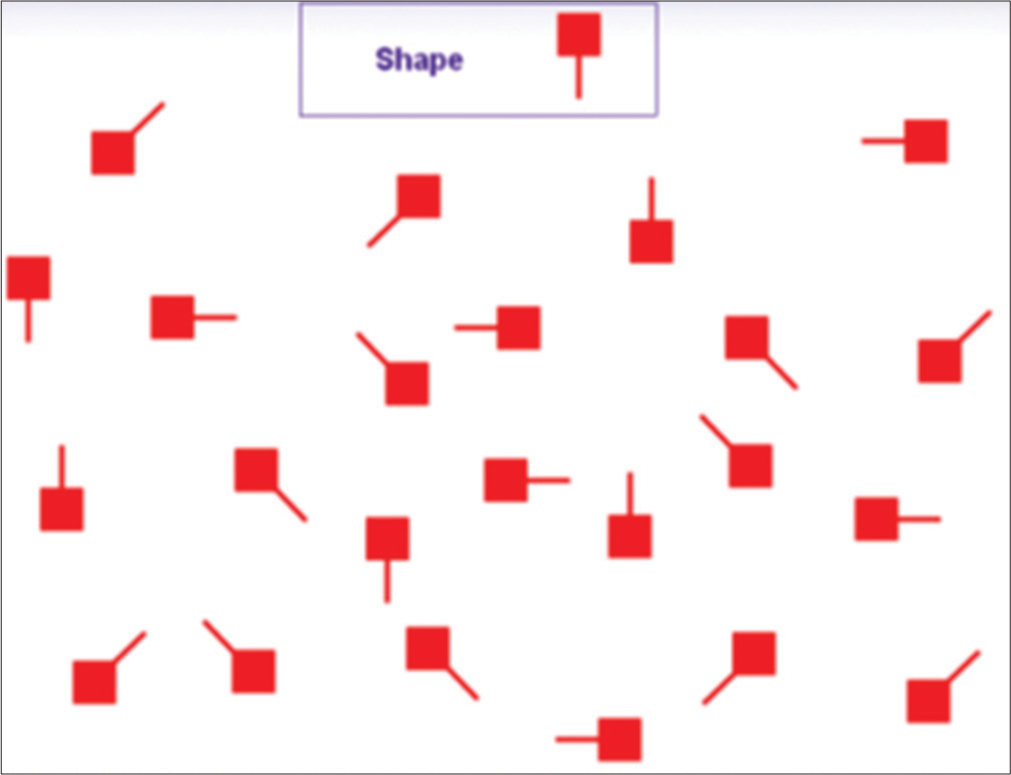
- This figure depicts the schematic representation of the visual attention task used in our neurocognitive battery.
Statistical analysis
Statistical analyses were conducted using the Statistical Package for the Social Sciences software.[23] The categorical variables were represented in proportions, and the mean and standard deviation were calculated for the continuous variables. The data was analyzed for normality using the Shapiro–Wilk test. The Chi-square and t-test were performed to analyze the differences in means of sociodemographic variables, ApoE ε4 genotype, and attention function. Linear regression models were used to examine the association between the ApoE genotype and attention scores. In the regression analysis, the ApoE genotype was taken as the predictor/exposure variable, and attention scores were the outcome variable.
Further, to examine the effect of age, gender, and education on the association between ApoE genotype and attention function, we made adjustments in the regression model. M odel 1 (unadjusted) included the direct association of the ApoE genotype, whereas model 2 was adjusted for ApoE and age. Model 3 included the effect of age and gender on the association between ApoE genotype and attention function, whereas model 4 included the effect of age, gender, and education on the association between ApoE genotype and attention. R square values, beta coefficients, and statistical significance (p < 0.05) were calculated for all the models.
RESULTS
Population characteristics
Out of the initial SANSCOG baseline dataset of 3013 subjects with ApoE data, 913 were excluded from the present study if they had missing data for at least one of the studied variables. Thus, the sample for analysis was 2100. The age range of the population was 45 to 96 years, with a mean age of 57.33 years (standard deviation [SD] = 9.15). In the stratified age group of 45–54 years, the mean age was 49.0 (SD = 2.64), for 55–64 years, it was 59.03 (SD = 2.82), and for 65 years and above, it was 70.08 (SD = 5.07). There was no significant gender difference between male participants (n = 941, 44.7%) and female participants (n = 1163, 55.3%). In the education demographic, the participants in the literate category were 1012 (48.1%), low literate were 380 (18.1%), and illiterate were 712 (33.8%), and these differences were not statistically significant [Table 1].
| Variables | ApoE genotype | |||
|---|---|---|---|---|
| No ε4 | Heterozygous ε4 | Homozygous ε4 | P | |
| Age | 57.25 (9.15) n=1767 | 57.89 (9.23) n=312 | 55.57 (7.97) n=21 | |
| 45–54 years | 787 | 136 | 9 | 0.702 |
| 55–64 years | 544 | 95 | 9 | |
| 65 years and above | 436 | 81 | 3 | |
| Gender | ||||
| Female | 985 | 168 | 8 | 0.232 |
| Male | 782 | 144 | 13 | |
| Education | ||||
| Illiterate | 601 | 106 | 4 | 0.435 |
| Low Literacy | 311 | 62 | 6 | |
| Literate | 855 | 144 | 11 | |
ApoE: Apolipoprotein E
ApoE genotype characteristics
The allele frequency data set showed that 1767 (84%) of the population did not have ApoE ε4, 312 (14.8%) were heterozygous, and 21 (1%) were homozygous for the ε4 allele [Table 2]. The mean attention score of the no ε4 group was 15.45 (SD = 3.71); the heterozygous ε4 group had a mean of 15.48 (SD = 3.81), whereas the homozygous ε4 group had a mean score of 15.57 (SD = 3.57). The above differences between the groups were not statistically significant (F = 0.895, P = 0.409).
| ApoE ε4 allele | ||
|---|---|---|
| Genotype | Frequency | Percentage |
| No ε4 | 1767 | 84.15 |
| Heterozygous ε4 | 312 | 14.85 |
| Homozygous ε4 | 21 | 1 |
| Total | 2100 | 100 |
ApoE: Apolipoprotein E
Model 1: Association between ApoE ε4 on attention
In the unadjusted linear regression model 1, we did not find an association between ApoE ε4 and attention function (F = [2, 7] = 0.896, P = 0.408) [Table 3, Figures 3 and 4].
| Predictors | Attention | Adjusted for age | Adjusted for age and gender | Adjusted for age, gender and education | ||||
|---|---|---|---|---|---|---|---|---|
| (Model 1) | (Model 2) | (Model 3) | (Model 4) | |||||
| β | P | β | P | β | P | β | P | |
| ApoE status | ||||||||
| Ref: No ε4 | ||||||||
| Heterozygous | 0.003 | 0.9 | 0.004 | 0.84 | 0.009 | 0.64 | 0.009 | 0.62 |
| Homozygous | 0.029 | 0.18 | 0.02 | 0.19 | 0.039 | 0.05 | 0.022 | 0.22 |
| Age groups (years) | ||||||||
| Ref: 45–54 | ||||||||
| 55–64 | −0.06 | 0.004 | −0.101 | 0.001 | −0.139 | 0.001 | −0.06 | 0.004 |
| 65 and above | −0.157 | 0.001 | −0.171 | 0.001 | −0.236 | 0.001 | −0.157 | 0.001 |
| Gender | ||||||||
| Ref: Female | ||||||||
| Male | −0.15 | 0.001 | −0.335 | 0.001 | −0.159 | 0.001 | ||
| Education | ||||||||
| Ref: Literate | ||||||||
| Illiterate | 0.474 | 0.001 | 0.227 | 0.001 | ||||
| Low Literate | −0.159 | 0.001 | 0.474 | 0.001 | ||||
ApoE: Apolipoprotein E
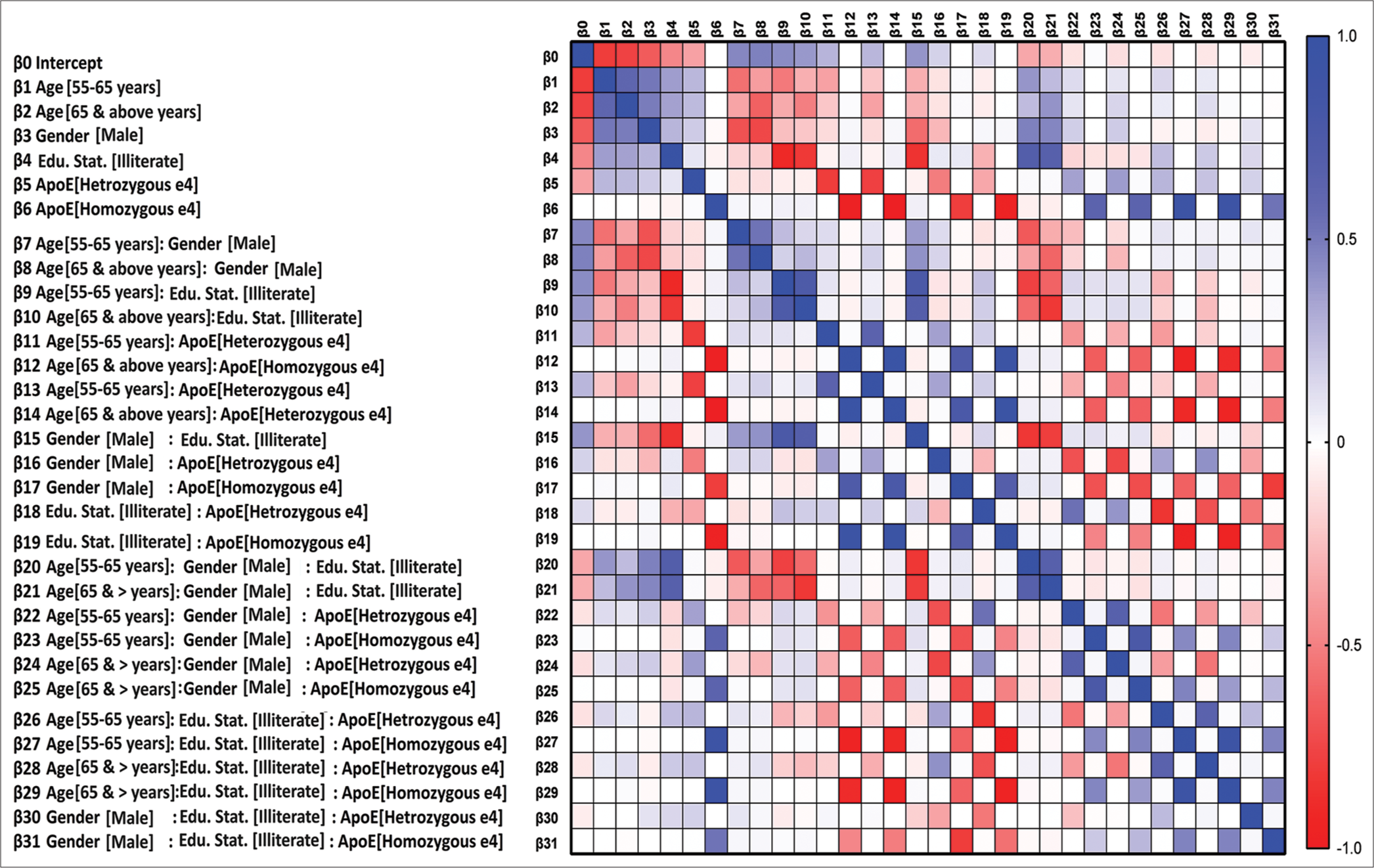
- This figure shows the heat map generated through model 4 (Apolipoprotein E adjusted for age, gender, and education). Note: Illiterate includes illiterate and low literate.
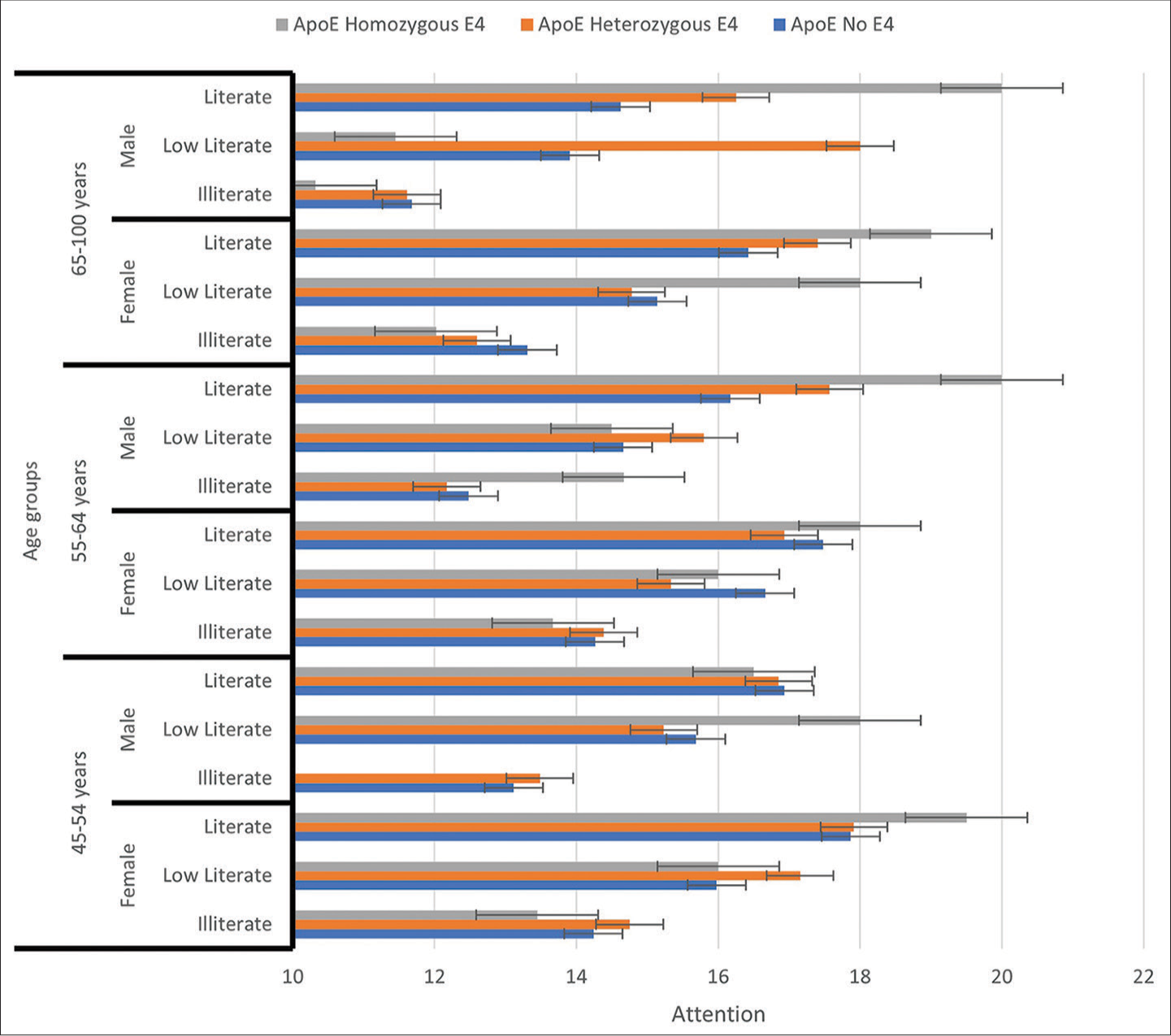
- This figure represents attention scores as a function of age, gender, and education status among no ε4, heterozygous ε4, and homozygous ε4 participants.
Model 2: Effect of ApoE ε4 on attention with age
In the partially adjusted model 2, ApoE ε4 with age was significantly associated with the attention scores. However, the variance in attention scores explained by ApoE ε4 genotype with age showed a low association (F [4, 7] = 14.51, P = 0.001, R2= 0.027, R2Adjusted = 0.025). Further, it was observed that attention scores decreased in the age group of 55–65 years (β = −0.10, P = 0.001) and 65 years and above (β = −0.171, P = 0.001) [Figure 4].
Model 3: Effect of ApoE ε4 on attention with age and gender
In model 3, we found that ApoE ε4, age, and gender explained a significant amount of the variance in attention scores (F [5, 7] = 65.69, P = 0.001, R2 = 0.135, R2Adjusted = 0.135). The model also showed that males had poorer attention (β = −0.33, P = 0.001) than females. After adjustment for gender, individuals in the age group 65 years and above (β = −0.236, P = 0.001) had poorer attention than those aged 55–64 years (β = −0.139, P = 0.001). Further, we found that homozygous ε4 female participants in the age groups 45–55 years and 55– 65 years had poorer attention than no ε4 and heterozygous ε4 males in the age group of 65 years and above [Figures 3 and 4].
Model 4: Effect of ApoE ε4 on attention with age, gender, and education
The fully adjusted model 4, wherein ApoE status, age, gender, and education were included, was statistically significant (F [7, 7] = 116.71, P = 0.001) and explained 27% of the variance in attention scores (R2 = 0.28, R2Adjusted = 0.278). Further, it showed that illiterate participants (β = −0.448, P = 0.001) had poorer attention function than those with low literacy (β = −0.138, P = 0.001), indicating the effect of education on the association between ApoE and attention. Further, all ApoE status (no ε4, homozygous ε4, and heterozygotes ε4) illiterates in the age group 45–55 years of both genders had poorer attention as compared to homozygous and heterozygous ε4 literates aged 65 years and above, of both genders [Figures 3 and 4].
DISCUSSION
This study examined the association between ApoE ε4 genotype and attentional function among aging adults without dementia from rural India. We found no significant association between ApoE ε4 genotype and attention function; however, it interacted with age, gender, and education to affect attention function in this rural Indian population, where older individuals (≥ 65 years), males, and illiterates had poorer attention scores than their respective counterpart groups.
The absence of an association between APOE ε4 genotype and attentional function in our study could be because our sample population comprised cognitively healthy individuals with a mean age of 57.33 years (SD = 9.15). In contrast, some recent studies among Caucasian populations have shown that ApoE ε4 status was associated with attentional deficits, which were observed among middle-aged and older adult populations.[24] A study from the U.S. showed that impairments in visuospatial attention were observed midlife in ApoE ε4 carriers, suggesting that attentional deficits may be an early clinical biomarker for AD.[25] Another study among dementia-free individuals from the U.K. revealed that ApoE ε4 carriers had a reduction in memory-guided attention.[26]
In our study, we found that the ApoE ε4 genotype with increasing age had poorer attentional performance (model 2), suggesting that the negative effects of ApoE ε4 on attention are likely to emerge in older ages. This is in line with the findings of a study among 240 adults with Down’s syndrome (a well-established risk for developing AD), which revealed that though there was no association between ApoE ε4 and attentional abilities, there was a significant association between ApoE status and age, wherin older individuals carrying ApoE ε4 showed poorer attentional ability than non-carriers.[27] Similarly, a recent longitudinal study[28] revealed an interaction between ApoE genotype and an increase in age on MMSE scores. Furthermore, the authors observed that the ApoE ε4 allele may accelerate the age-related deterioration in language ability, executive function, and attention, which are presumed to be the three primary cognitive domains affected in moderate cognitive impairment and early stages of AD.
On adjusting for gender in our study (model 3), we found that males had poorer attention as compared to females. While several earlier studies have shown gender differences in cognitive performance, only very few demonstrated a qualitative difference in attentional tasks. While males profit from an invalid cue compared to the no-cue control condition, females demonstrate greater costs to an invalid cue.[29] Another study on how gender and ApoE affect P3a (an electroencephalographic, EEG component that is considered as an index of brain activity related to attention) in amnestic MCI (aMCI) and healthy controls (HC) revealed that in both groups, novelty P3a amplitudes were lower in males than in females.[30]
Another important finding in our study was the effect of education on the association between ApoE and attention, where it was observed that illiterate participants had poorer attentional performance than those with low literacy. This could be explained by the “cognitive reserve” hypothesis which suggests that people who have cognitively stimulating experiences through better educational attainments have better cognitive function. Arenaza-Urquijo et al. demonstrated that in cognitively healthy participants, ApoE ε4 carriers with higher education had better metabolism in the frontal and temporal regions in fluorodeoxyglucose-positron emission tomography studies (middle temporal lobe metabolism among higher educated carriers were comparable to that in non-carriers). The findings of the above-mentioned study indicate that education could possibly serve as a protective factor against the adverse cognitive changes seen in ApoE ε4 carriers.[31]
Our study is one of the few studies that have specifically examined the association of ApoE ε4 status on attentional functioning and to the extent that we know, there are no such studies from a rural Indian, dementia-free, aging population. The strengths of our study include a large sample size and a relatively homogeneous rural population. This, in addition to the wide age range (45–96 years) of our participants, enabled us to classify them into three age groups, so that the interaction of age with ApoE ε4 status could be examined. Further, most of the previous studies have used short screening tests to measure global cognition, whereas we have specifically assessed attentional function using a domain-specific, computerized, and neurocognitive test battery.
There are certain limitations of our study. One is the cross-sectional study design, which prevents us from making any causal associations. Another is the small number of participants with homozygous ApoE ε4 alleles (n = 21, 1%). Further, ApoE ε4 could have an effect on cognitive performance by playing a medicating role on other risk factors of dementia, such as cardiovascular risk factors, which we have not taken into account in our study.
We plan to follow up with the participants in this study with serial monitoring of their attentional performance to find out if ApoE ε4 carriers have a faster rate of decline in their attention functions. Further, it will also be interesting to compare the trajectories of attentional decline with the trajectories of decline in other cognitive domains to see if ApoE ε4 has a differential effect on particular cognitive domains only. In addition, following up a subset of these participants who subsequently develop MCI, to see how their ApoE ε4 status influences further cognitive deterioration and the rate of progression to dementia, could also yield useful insights into the effect of ApoE ε4 genotype on specific cognitive domains.
CONCLUSION
In sum, ApoE genotype alone was not cross-sectionally associated with attention. However, it altered the association of age, gender, and education with attention function. Longitudinal monitoring of this population is needed to determine if ApoE ε4 genotype is linked to accelerated cognitive decline with aging.
Acknowledgments
We are grateful to the volunteers who participated in the SANSCOG study. We acknowledge all members of the SANSCOG study team for their valuable contributions to various aspects of the SANSCOG study.
Data availability statement
The data that supports the findings of this study is available from the corresponding author, JSS, upon reasonable request.
Ethical approval
The study is approved by the Institutional Ethics Committee of Centre for Brain Research, Indian Institute of Science, Bangalore, number CBR/42/IEC/2022-23, dated 21st November 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
The SANSCOG study is funded through the Centre for Brain Research, Indian Institute of Science, India by Pratiksha Trust.
References
- The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: Results from Apo Europe. Eur J Hum Genet. 2002;10:841-50.
- [CrossRef] [PubMed] [Google Scholar]
- Apolipoprotein E receptors mediate the effects of beta-amyloid on astrocyte cultures. J Biol Chem. 2000;275:33974-80.
- [CrossRef] [PubMed] [Google Scholar]
- Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644-51.
- [CrossRef] [Google Scholar]
- Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501-18.
- [CrossRef] [Google Scholar]
- Better lipids to power next generation of mRNA vaccines. Science. 2022;376:680-1.
- [CrossRef] [Google Scholar]
- A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer's disease in African Ancestry. PLoS Genet. 2022;18:e1009977.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of estrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429-32.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of APOE and TOMM40 allele frequencies in the Japanese population. Alzheimers Dement (N Y). 2017;3:524-30.
- [CrossRef] [PubMed] [Google Scholar]
- Apolipoprotein E ε4 prevalence in Alzheimer's disease patients varies across global populations: A systematic literature review and meta-analysis. Dement Geriatr Cogn Disord. 2011;31:20-30.
- [CrossRef] [Google Scholar]
- Apolipoprotein E polymorphism and dementia: A hospital-based study from Southern India. Dement Geriatr Cogn Disord. 2011;30:455-60.
- [CrossRef] [PubMed] [Google Scholar]
- Association of apolipoprotein E genetic variation in Alzheimer's disease in Indian population: A meta-analysis. Am J Alzheimers Dis Other Demen. 2014;29:575-82.
- [CrossRef] [PubMed] [Google Scholar]
- APOE2: Protective mechanism and therapeutic implications for Alzheimer's disease. Mol Neurodegener. 2020;15:63.
- [CrossRef] [PubMed] [Google Scholar]
- APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J Gerontol A Biol Sci Med Sci. 2014;69:379-86.
- [CrossRef] [PubMed] [Google Scholar]
- Changing demography and the challenge of dementia in India. Nat Rev Neurol. 2021;17:747-58.
- [CrossRef] [PubMed] [Google Scholar]
- APOE ε4 allele is associated with worse performance in memory dimensions of the mini-mental state examination: The Bambuí cohort study of aging. Int J Geriatr Psychiatry. 2015;30:573-9.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of cognitive training on domains of attention in older adults with mild cognitive impairment and mild dementia: A meta-analysis of randomised controlled trials. J Glob Health. 2023;13:4078.
- [CrossRef] [Google Scholar]
- Association of apolipoprotein E genetic variation in Alzheimer's disease in Indian population: A meta-analysis. Am J Alzheimers Dis Other Demen. 2014;29:575-82.
- [CrossRef] [PubMed] [Google Scholar]
- Approaches to engage an aging, rural cohort in Southern India during the COVID-19 crisis and the psychological impact of COVID-19 in this cohort. Alzheimers Dement. 2022;19:2460-8.
- [CrossRef] [PubMed] [Google Scholar]
- Srinivaspura aging, neuro senescence and COGnition (SANSCOG) study: Study protocol. Alzheimers Dement. 2022;19:2450-9.
- [CrossRef] [PubMed] [Google Scholar]
- COGNITO: Computerized assessment of information processing. J Psychol Psychother. 2014;4:136.
- [Google Scholar]
- COGNITO (Computerized assessment of adult information processing): Normative scores for a rural Indian population from the SANSCOG study. Alzheimers Dement. 2022;19:2433-42.
- [CrossRef] [PubMed] [Google Scholar]
- APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer's disease: A systematic review. Alzheimers Res Ther. 2020;12:141.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of apolipoprotein E genotype on visuospatial attention dissipates after age 80. Neuropsychology. 2009;23:81-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neural dynamics supporting auditory long-term memory effects on target detection. Neuroimage. 2020;218:116979.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of genetic variants associated with cognitive impairment and depressive symptoms in Parkinson's disease. Acta Neuropsychiatr. 2019;32:10-22.
- [CrossRef] [PubMed] [Google Scholar]
- Association of APOE genotype with heterogeneity of cognitive decline rate in alzheimer disease. Neurology. 2021;96:e2414-e2428.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for gender differences in visual selective attention. Pers Individ Dif. 2007;43:597-609.
- [CrossRef] [Google Scholar]
- Effects of gender and apolipoprotein E on novelty MMN and P3a in healthy elderly and amnestic mild cognitive impairment. Front Aging Neurosci. 2018;10:256.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between years of education and APOE e4 status on frontal and temporal metabolism. Neurology. 2015;85:1392-9.
- [CrossRef] [PubMed] [Google Scholar]







