Translate this page into:
Sporadic cerebral amyloid angiopathy: An important cause of cerebral hemorrhage in the elderly
Address for correspondence: Dr. Shahina Bano, Department of Radiodiagnosis, Govind Ballabh Pant Hospital and Maulana Azad Medical College, New Delhi-110 002, India. E-mail: dr_shahinaindia@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cerebral amyloid angiopathy (CAA) is an important cause of primary intracerebral hemorrhage (PICH) in the elderly. Although there are no pathognomic clinical features of CAA-related PICH, the association of white matter changes with lobar, recurrent, or multiple simultaneous hemorrhages in older patients should raise the suspicion of its diagnosis. A definitive diagnosis of CAA requires pathologic examination of the affected tissue. However, with modern imaging techniques, it is possible to diagnose the “probable CAA” in patients presenting with PICH. Gradient-echo magnetic resonance imaging is a very sensitive, noninvasive technique for identifying microhemorrhages in life. The diagnosis of CAA is important because of the likely implication it has on future management targeted to reduce risk of future bleeding.
Keywords
Cerebral amyloid angiopathy
computed tomography
magnetic resonance imaging
Introduction
Cerebral amyloid angiopathy (CAA), also known as congophilic angiopathy or cerebral amyloidosis, is a clinicopathologic condition resulting from the extracellular deposition of an amorphous eosinophilic substance (a fibrillar protein, amyloid) in the walls of small- and medium-sized arteries. The amyloid material is only found in brain and therefore this disease is not related to any other form of systematic amyloidosis. Although CAA can manifest in several ways, the most serious manifestation of CAA in older people is the rupture of cerebral vessels, leading to intracerebral hemorrhage, accounting for about 10% of all types of PICH.[1] The incidence of PICH increases with age, and is rare in those younger than 55 years. Although CAA most commonly occurs in sporadic form in the elderly, numerous familial forms also occur in the younger age group.[2] It is difficult to make a definitive diagnosis of CAA in life, as it requires pathologic examination of the brain tissue. However, recent developments in brain imaging techniques have greatly helped in allowing a diagnosis of “probable CAA” to be made, as per Boston Criteria for Diagnosis of CAA-related hemorrhage[3] [Table 1]. CAA may occur in the absence of clinical and/or pathologic evidence (eg, amyloid plaques) of Alzheimer's disease. In a setting of diffusely increased vascular fragility, microhemorrhages could occur when they are not clinically apparent, but should be detected by magnetic resonance imaging (MRI). The sensitivity of MRI for hemosiderin provides unique opportunity to identify old microhemorrhages. Although most studies define microhemorrhages as being smaller than 5 mm in diameter, an upper limit of 10 mm is sometimes used.[4] There is also a high prevalence of white matter lesions on CT and MRI in patients with lobar PICH and CAA.
Case Report
A 66-year-old normotensive, nondiabetic man, presented to our hospital with clinical features of stroke. The patient had mild, progressive cognitive decline, and few episodes of transient ischemic attack beginning few weeks before the major stroke, for which he had not taken any medication. His blood coagulation profile was normal.
CT and MR images of brain were obtained 2 weeks after the stroke. A plain axial CT image [Figure 1] showed right parietal lobe hematoma with diffuse cerebral white matter hypodensities. Corresponding axial T1-weighted MR image [Figure 2] demonstrated subacute nature of right parietal hematoma. T2-weighted [Figure 3] and FLAIR [Figure 4] MRI images showed extensive supratentorial white matter hyperintensities favoring CAA-related inflammatory leukoencephalopathy. The white matter changes were diffuse, concomitant, confluent (grade 3), more pronounced on the right side and involved both periventicular deep white matter and subcortical U-fibers. Multiple punctate (grade 1) white matter hyperintensities were also visible in bilateral frontoparietal and left basal ganglia region. T2*-weighted gradient-echo (GRE) MR images [Figure 5] showed multiple focal hemosiderin deposits (old microbleeds) in the cortical and corticosubcortical location of right frontotemporal lobe, and solitary fresh microbleed in the left high posterofrontal region, as signal void areas, giving a characteristic “blooming effect.” In addition, linear areas of signal void visible in the vicinity of the primary lobar hemorrhage, and right frontal lobe suggested subarachnoid hemosiderosis. Although diffusion weighted imaging (DWI) and the corresponding apparent diffusion coefficient (ADC) mapping [Figure 6] revealed restricted diffusion within large lobar subacute bleed, but no other area in the brain demonstrated diffusion restriction, excluding acute infarct. Mild age-related generalized brain atrophy was also evident.
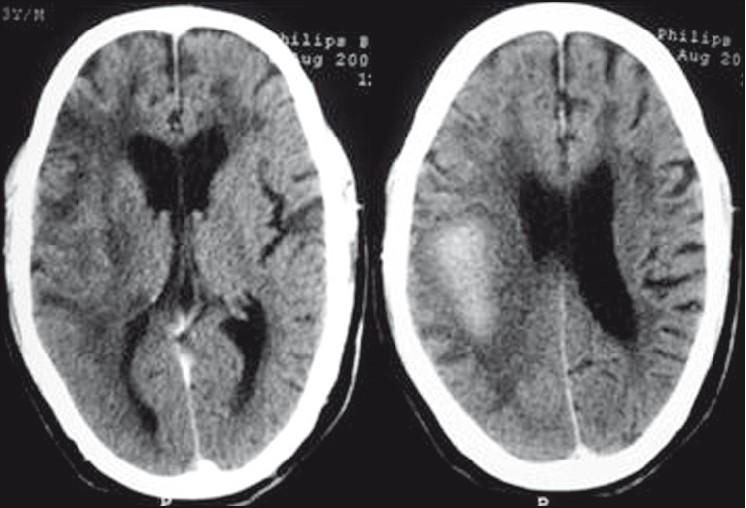
- Plain axial CT image reveals large, right parietal lobe hematoma causing mild compression of posterior part of the body of ipsilateral lateral ventricle; and diffuse white matter hypodensities (leukoaraiosis)
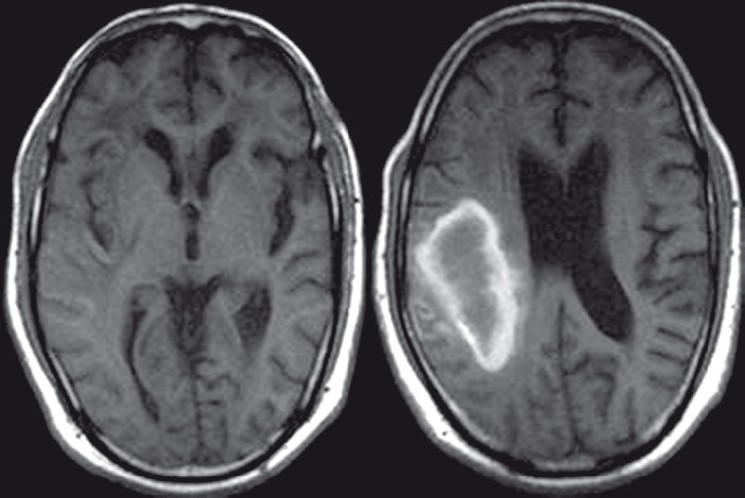
- Axial T1-weighted MR image demonstrates subacute right parietal hematoma and associated white matter hypointensities. Mild age-related generalized brain atrophy with prominence of cerebrospinal fluid spaces is also evident
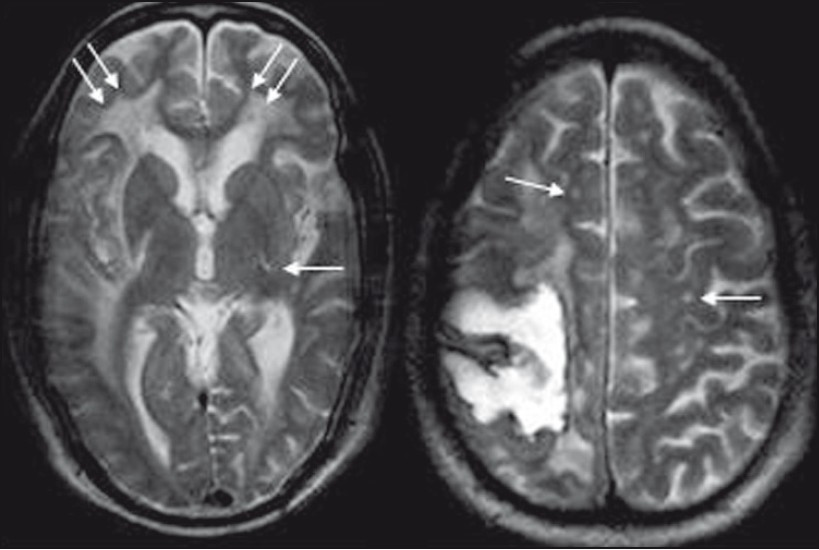
- T2-weighted axial image reveals diffuse, asymmetrical, confluent (grade 3) supratentorial white matter hyperintensities, being more pronounced on the right side, involving both periventicular deep white matter and subcortical U-fibers (double arrow). Multiple punctate (grade 1) hyperintense foci (single arrow) are also visible in bilateral frontoparietal and left basal ganglia region
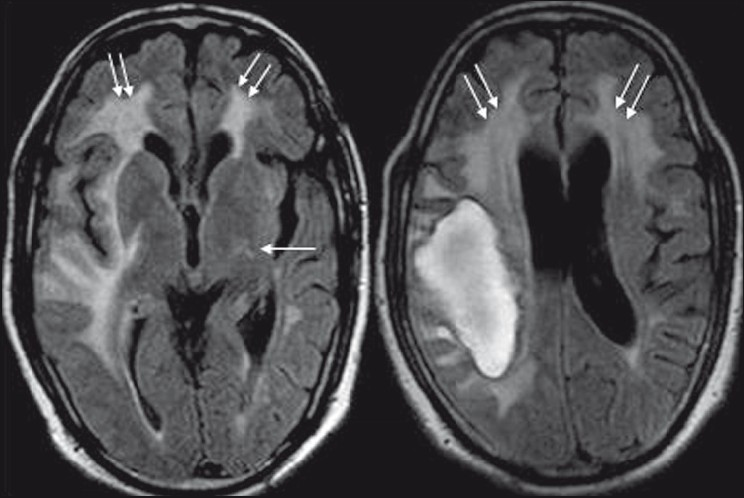
- Axial FLAIR sequences demonstrate the lobar hematoma with irregular borders, and supratentorial white matter hyperintensities as seen in T2-weighted images
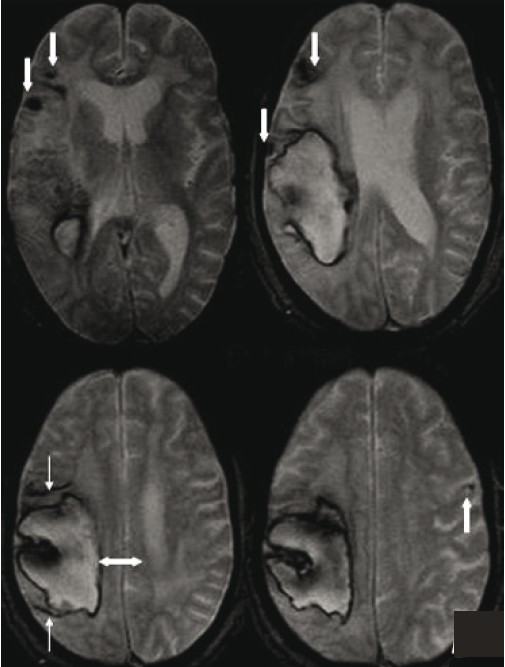
- T2FNx01-weighted gradient-echo MR images show multiple focal hemosiderin deposits (old microbleeds) in the cortical and corticosubcortical location of right frontotemporal lobe (thick downarrow), and solitary fresh microbleed in the left high posterofrontal region (thick up-arrow), seen as signal void areas (blooming). In addition, linear areas of signal void (thin arrow) in the vicinity of large primary lobar hemorrhage (double arrow) suggest subarachnoid hemosiderosis
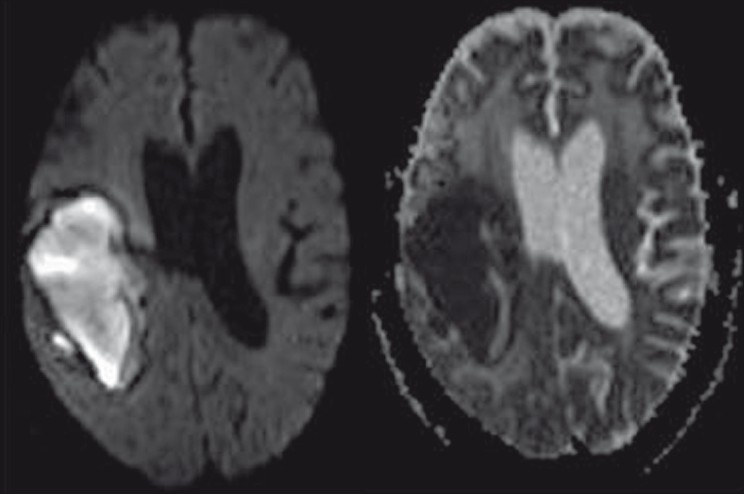
- DWI and the corresponding ADC mapping reveal restricted diffusion within the subacute bleed. No other area in the brain parenchyma demonstrates diffusion restriction, thereby excluding acute cerebral infarct
Other potential causes of spontaneous hemorrhage, such as cavernous angioma or other vascular malformations, were excluded using MR angiography. In addition, hemorrhages in locations more typical of hypertensive intracerebral hemorrhage (ICH), such as the basal ganglia and thalamus, were also excluded. Based on the above clinical and imaging findings and absence of other causes of cerebral hemorrhage, the patient fulfilled the Boston criteria for diagnosis of probable CAA.
In our case, the patient was managed conservatively. He was given diuretics to reduce the brain edema, and corticosteroid for coexisting cerebral amyloid inflammatory vasculopathy responsible for leukoencephalopathy and subacute progressive cognitive decline. After a period of rehabilitation, the patient did show some improvement but was not able to live independently, and was discharged to be cared for by his family.
DISCUSSION
Spontaneous ICH is primarily caused by the rupture of small- and medium-sized arteries. Hypertension-induced fibrohyalinosis and deposition of β-amyloid within the walls of small vessels are important causes of increased vascular fragility.[15] In addition to creating a higher risk for ICH, both types of angiopathy favor the development of ischemic damage to the brain. Tissue changes, such as leukoaraiosis and a lacunar state, are therefore frequently observed in patients suffering from ICH, and their presence has been suggested to indicate a higher risk for cerebral hemorrhage.[4] In a study that compared the clinical features of PICH due to CAA and those due to hypertension, the features of CAA-related PICH included lobar distribution affecting mainly lobar superficial areas, multiplicity of hemorrhage, bilaterality, repeated episodes, lobulated appearance, rupture into the subarachnoid space, and secondary intraventricular hemorrhage from the lobar hemorrhage.[5] The location of cerebral microbleeds may help to determine the underlying cause of intracerebral hemorrhage. An exclusively corticosubcortical distribution suggests amyloid angiopathy, whereas deeper microbleeds in the basal ganglia/thalami, brainstem, and cerebellum suggest hypertensive small vessel disease. Thus patients with CAA tend to have corticosubcortical/lobar microbleeds and lobar hemorrhages, whereas patients with hypertensive disease tend to have deep microbleeds and deep hemorrhages.[56] The severity of a white matter hyperintensity (leukoencephalopathy) on T2W sequences was graded as punctuate (grade 1), early confluent (grade 2), or large confluent (grade 3).[7] The leukoencephalopathy in CAA may be categorized as leukoencephalopathy with sparing of U-fibers or leukoencephalopathy with the involvement of U-fibers. Leukoencephalopathy with sparing of U-fibers is due to ischemia caused by diffuse narrowing of penetrating cortical vessels resulting from β-amyloid deposition in the adventitia, whereas leukoencephalopathy that extends to involve U-fibers and is associated with edema, is related to perivascular inflammation secondary to β-amyloid-induced vasculopathy. Accumulating evidence suggests that white matter changes in CAA increases over time and is linked to worsening of cognitive impairment.8
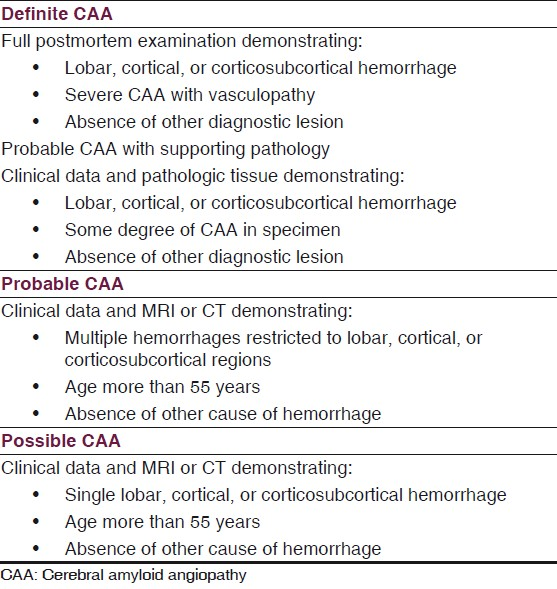
A set of clinical diagnostic criteria have been proposed by the Boston group in an attempt to unify the diagnosis of CAA to be definite, probable with pathology, probable without pathology and possible.[3] Box 1 provides these criteria. Recently, Linn et al have proposed a modified Boston criterion, in which they have suggested that inclusion of superficial siderosis (SS) as a criterion increases the sensitivity of the classic Boston criteria for CAA-related hemorrhage without the loss of specificity. They have found that SS occurs with high prevalence in CAA and is rare in the non-CAA form of intracerebral hemorrhages.[9]
CT and MRI of brain are commonly used to diagnose CAA. Although useful in diagnosing PICH, CT provides limited information regarding the underlying cause, and in the detection of chronic asymptomatic microhemorrhages. In this context, attention is focused on MRI because of its potential to reveal the residue of intracerebral hemorrhage throughout life, and even subtle white matter changes. After an ICH, hemosiderin remains stored in macrophages and, because of its magnetic properties, leads to focal dephasing of the MR signal, causing hemosiderin-containing areas to appear dark on T2-weighted spin-echo sequences. This effect may be further enhanced by the use of imaging techniques with high sensitivity for differences in magnetic susceptibility, such as the GRE sequence.[10] The susceptibility-weighted imaging (SWI) has been used to evaluate the presence of microhemorrhages, and is found to be more sensitive than conventional GRE techniques for the detection of blood products.[11] The differentiation of microhemorrhages from calcifications (old calcified granulomas) can sometimes be difficult in T2*W images without CT scans. Phase images of SWI may help.
Moreover, MR evidence of past microbleeds appears to be a better predictor of a patient's risk for hemorrhage than are clinical findings, such as hypertension, or CT changes of leukoaraiosis. In this context, MRI might help in selecting patients for different types of secondary prevention of stroke. The major concern in the survivors of CAA-related PICH is the recurrent bleeding. A recurrence rate of 10% per year has been reported.[12] The recurrence of hemorrhage carries particularly poor prognosis with inhospital mortality of up to 70%.[12] Some authors have suggested routine use of GRE MRI sequences to detect microbleeds in older people to avoid potentially dangerous anticoagulant or antiplatelet therapy.[6] However, the SWI has been found to be more sensitive than conventional GRE sequences in identifying CAA-related microhemorrhages.[11]
The usefulness of brain biopsy in diagnosis of CAA has also been evaluated by many authors. Steven et al. took 28 cortical biopsy samples from postmortem brains with known CAA. Microscopically, these samples demonstrated the presence of vascular amyloid as a sensitive marker for CAA-related hemorrhage. Thus the examination of biopsies taken from cortical tissues also plays an important role in diagnosing this condition.[13]
Currently, there is no specific therapy available for treating CAA. However, recent advances in immunopathology and pathogenesis of CAA have helped to develop a specific antiamyloid drug, Cerebril (Neurochem Laval, Canada), which has shown to reduce amyloid deposition and formation. At present, this drug is under phase 3 trial to prove its efficacy.[14] The management of CAA-related intracranial hemorrhage is identical to the standard management of ICH. Treatment of hypertension and other vascular risk factors, such as hypercholesterolemia, is usually advised. According to a recent progress trial, perindopril, an antihypertensive drug has shown reduction in CAA-related ICH by 77% over a follow-up period of 3.9 years.[15] Reduced consumption of alcohol is also an important part of secondary prevention. The coexisting vasculitis and cognitive decline can be treated with corticosteroids and cyclophosphamide. Cessation of cerebral hemorrhage recurrence associated with corticosteroid treatment in patients with CAA has also been documented in the literature.[16] Surgical evacuation of hematoma is indicated to lower the intracranial pressure, especially when hematoma causes significant mass effect and predisposes to herniation.[17] The anticoagulant and antiplatelet medications should be avoided in patients with CAA, particularly in elderly individuals with atrial fibrillation, because of high bleeding rates associated with the use of these agents. Several studies have shown that low-dose aspirin (eg, 75–150 mg) is highly effective in secondary prevention of intracranial bleed and risk of ischemic stroke.[1819]
We conclude that symptomatic PICH is frequently preceded by microangiopathy-related ischemic damage and silent microhemorrhages as seen in our case. Such an MR finding certainly favors an angiopathic cause for the bleeding and thus may obviate more invasive diagnostic procedures. The burden of asymptomatic cerebral microhemorrhages (both old and fresh) detectable by GRE MRI in patients with lobar PICH related to CAA is a good predictor of hemorrhage recurrence, and therefore highlights the importance of secondary prevention in CAA-related PICH.
Source of Support: Nil
Conflict of Interest: None declared.
References
- A prospective study of acute cerebrovascular disease in the community: The Oxfordshire community Stroke Project – 1981-86: Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:16-22.
- [Google Scholar]
- Familial cerebral amyloid angiopathy related to stroke and dementia. Amyloid. 2001;8:36-42.
- [Google Scholar]
- Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology. 2001;56:537-9.
- [Google Scholar]
- Study of clinical features of amyloid angiopathy hemorrhage and hypertensive intracerebral hemorrhage. J Zhejiang Univ Sci. 2004;5:1262-9.
- [Google Scholar]
- Petechial hemorrhages accompanying lobar hemorrhage: Detection by gradient-echo MRI. Neurology. 1996;46:1751-4.
- [Google Scholar]
- MR signal abnormalities at 1.5T in Alzheimer's dementia and normal aging. AJNR Am J Neuroradiol. 1987;8:421-6.
- [Google Scholar]
- Cerebral amyloid angiopathy: CT and MRI imaging findings. Radiographics. 2006;26:1517-31.
- [Google Scholar]
- Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346-50.
- [Google Scholar]
- MRI of cerebral microhemorrhages: Pictoral essay. AJR Am J Roentgenol. 2007;189:720-5.
- [Google Scholar]
- Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007;28:316-7.
- [Google Scholar]
- Pattern of recurrence of intracerebral hemorrhage. Semin Cerebrovasc Dis Stroke. 2005;5:168-71.
- [Google Scholar]
- Diagnosis of cerebral amyloid angiopathy sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418-22.
- [Google Scholar]
- Safety Data Presented for Cerebril at Neurology Meeting. Biotechnol Healthc. 2005;2
- [Google Scholar]
- Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: The PROGRESS trial. Stroke. 2010;41:394-6.
- [Google Scholar]
- Cessation of cerebral hemorrhage recurrence with corticosteroid treatment in a patient with cerebral amyloid angiopathy. Amyloid. 2000;7:284-8.
- [Google Scholar]
- Outcome of cerebral amyloid angiopathic brain hemorrhage. Acta Neurochir (Wien). 2008;150:889-95.
- [Google Scholar]
- Antithrombotic prophylaxis in elderly patients with atrial fibrillation. Semin Thromb Hemost. 2009;35:548-53.
- [Google Scholar]
- Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710-6.
- [Google Scholar]






