Translate this page into:
A Sporadic Cisternal Cystic Oculomotor Schwannoma—Case Report and Review of Literature
Sanjay Honavalli Murali, MBBS, MS, MCh Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical Sciences (SCTIMST) Trivandrum 695011, Kerala India skullshake@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Schwannoma arising from a pure motor cranial nerve in sporadic cases is a rare finding. We report adult female patient presented with a seizure without oculomotor palsy. On evaluation, she was diagnosed with cystic oculomotor schwannoma and underwent surgery. She developed oculomotor palsy postoperatively. Here, we describe a rare sporadic cystic oculomotor schwannoma.
Keywords
cystic schwannoma
oculomotor schwannoma
cisternal schwannoma
Introduction
Intracranial schwannoma comprises 7 to 8% of intracranial tumors. Most commonly, they involve the vestibular and trigeminal nerves, followed by the lower cranial nerves.1 There are many case reports of oculomotor schwannomas; however, purely cystic oculomotor schwannomas are mentioned only in three case reports.2
Oculomotor schwannomas are commonly associated with neurofibromatosis, but sporadic cisternal purely cystic oculomotor schwannoma is still a rarely reported entity. Our case report intends to impress upon neurosurgeons to consider this as a possible differential when confronted with a similar radiological picture.
Case Report
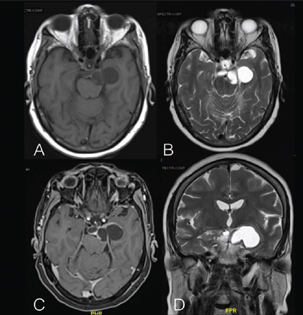
A 71-year-old female patient without any comorbidities and no significant family history presented to us with a history of one episode of seizure. Her clinical examination did not reveal any cranial nerve palsy or restriction of eye movement. She had no markers suggestive of neurofibromatosis. Her neurological evaluation was normal. She underwent imaging that showed a (Fig. 1) 2.6 cm (anteroposterior)x 4.3 cm (transverse)x 2.8 cm (sagittal) multiloculated thick-walled cystic lesion with T2-weighted imaging (T2WI) showing hyperintensity and T1WI showing hypointensity, located in the left middle cranial fossa, suprasellar region, and left interpeduncular region. The lesion was enhancing on contrast. It was abutting the left optic radiation, pituitary infundibulum, and the basilar trunk. Posterior communicating artery was displaced medially, and superior cerebellar artery was displaced inferiorly. The lesion showed no restricted diffusion. Rest of the parenchyma was normal. The trigeminal nerve was seen separate from the lesion. Our radiological differential diagnoses included ganglioglioma, schwannoma, pilocytic astrocytoma, and less likely craniopharyngioma and metastasis.
-
Fig. 1 (A) T1-weighted imaging (T1WI) axial section magnetic resonance imaging (MRI) shows hypointense and (B) T2WI axial and (D) T2WI coronal section MRI shows hyperintense cystic lesion involving interpeduncular cistern, suprasellar cistern, crural cistern, extending to the supratentorial region with mass effect on the medial temporal lobe. (C) Contrast axial section MRI showing the enhanced cyst wall.
Fig. 1 (A) T1-weighted imaging (T1WI) axial section magnetic resonance imaging (MRI) shows hypointense and (B) T2WI axial and (D) T2WI coronal section MRI shows hyperintense cystic lesion involving interpeduncular cistern, suprasellar cistern, crural cistern, extending to the supratentorial region with mass effect on the medial temporal lobe. (C) Contrast axial section MRI showing the enhanced cyst wall.
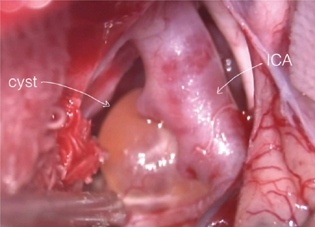
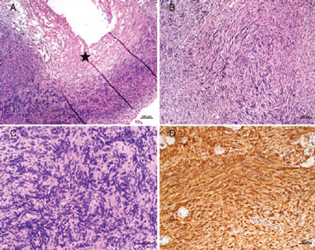
The lesion was situated extra-axially and confined to the cistern; the closest route was via the trans-sylvian approach with a pterional craniotomy. Intraoperatively, the cyst wall was thickened and firm. On opening, the cyst revealed straw-colored fluid within. The cyst wall (Fig. 2) was adherent with the basilar artery and the left posterior communicating artery. We were not able to achieve gross total decompression as even slight traction induced cardiovascular instability due to trigemino-cardiac reflex. It was decided to leave behind part of the lesion (Fig. 3). Third cranial nerve could not be identified separately from the lesion. Postoperatively, the patient developed oculomotor palsy in form of ptosis, restriction of adduction, elevation and depression of the left eye, and anisocoria with left pupillary dilatation. Histopathological examination showed a tumor arising from a nerve (Fig. 4A) comprising cellular (Antoni A, Fig. 4B) and hypocellular (Antoni B) areas composed of sheets of spindle cells with wavy nuclei displaying nuclear palisading with formation of Verocay bodies (Fig. 4C). The spindle cells were positive for S100 protein (Fig. 4D). These morphological features were classical of schwannoma.
-
Fig. 2 Intraoperative image (trans-sylvian view) showing cystic lesion encasing internal carotid artery (ICA) and its branches. The medial component can be seen through the left opticocarotid recess
Fig. 2 Intraoperative image (trans-sylvian view) showing cystic lesion encasing internal carotid artery (ICA) and its branches. The medial component can be seen through the left opticocarotid recess
-
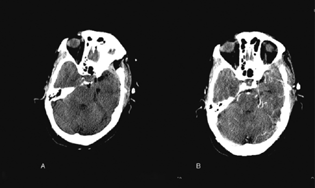
Fig. 3 (A) Postoperative plain computed tomographic scan and (B) postoperative contrast image showing a small enhancing residual above the tentorium near the hippocampus.
Fig. 3 (A) Postoperative plain computed tomographic scan and (B) postoperative contrast image showing a small enhancing residual above the tentorium near the hippocampus.
-
Fig 4 Histopathological examination showed a tumor arising from a nerve (A) comprising cellular (Antoni A, B) and hypocellular (Antoni B) areas composed of sheets of spindle cells with wavy nuclei displaying nuclear palisading with formation of Verocay bodies (C). The spindle cells were positive for S100 protein (D). These morphological features were classical of schwannoma.
Fig 4 Histopathological examination showed a tumor arising from a nerve (A) comprising cellular (Antoni A, B) and hypocellular (Antoni B) areas composed of sheets of spindle cells with wavy nuclei displaying nuclear palisading with formation of Verocay bodies (C). The spindle cells were positive for S100 protein (D). These morphological features were classical of schwannoma.
The postoperative scan revealed a small residual in the right peri mesencephalic cistern. She is under follow-up.
Discussion
Schwannoma arises from the Schwann cells that surround peripheral nerves and grows expansively. They are extra-axial lesions and are usually associated with neurofibromatosis. However, sporadic schwannomas are seen, but usually involve vestibular, trigeminal, and lower cranial nerves. Oculomotor schwannoma is a rare intracranial tumor.3 The first case was reported by Kovas in 1927 in an autopsy. After that multiple cases have been reported. To our knowledge, 39 cases have been reported till date.4 We could find only three case reports of purely cystic oculomotor schwannoma in literature and our case might be the fourth one.5
The third nerve traverses the middle cranial fossa in its course from the midbrain to the superior orbital fissure. It exits the brainstem in the interpeduncular fossa between superior cerebellar artery and posterior cerebellar artery and courses toward the ipsilateral uncus and pierces the wall of the cavernous sinus. It travels along with the fourth and fifth cranial nerves in the lateral wall of the cavernous sinus and enters the superior orbital fissure and divides into two branches. The superior branch supplies the superior rectus and the levator palpebrae superioris and the inferior branch supplies the medial and inferior rectus along with the inferior oblique extraocular muscles. The inferior branch also carries parasympathetic fibers to the ciliary ganglion. A schwannoma can arise anywhere along the course of the third nerve with the most common location being in the cistern proximally near the interpeduncular fossa.6 7
Oculomotor schwannoma usually presents with partial or complete third nerve palsy causing diplopia. It can also present with other symptoms due to its slow-growing nature and mass effect on surrounding structures like headache and periorbital pain due to trigeminal nerve compression, visual disturbance due to optic nerve compression, seizure due to medial temporal cortical irritation, and gait disturbance due to pressure on the cerebral peduncles. It can also present without any symptoms attributable to the third cranial nerve as in our case. In our case, the patient did not have any diplopia or visual disturbance but presented with a single episode of seizure. The magnetic resonance imaging showed the origin of the tumor within the interpeduncular area and extension toward uncus around the tent that lead us to put oculomotor schwannoma as a differential postoperatively. The correct diagnosis for such a rare tumor may not be possible preoperatively always.6 7 8 9 10
Though benign in nature, surgical excision is the treatment of choice for such cases. The choice of approach depends on the location of the lesion. Cisternal schwannoma can be better approached through pterional craniotomy and trans-sylvian route as it provides a wide corridor for tumor excision. In our case, we preferred the same. Oculomotor schwannoma is sometimes difficult to remove totally as it can convert partial third nerve palsy into a complete one. In our case, the patient did not have a preoperative deficit, but after surgery, she developed third nerve palsy. Due to the cystic component, we did not consider radiosurgery.11 12 13
Trigemino-cardiac reflex14 can occur during stimulation of any sensory branches of the trigeminal nerve and presents as parasympathetic excitation resulting in bradycardia, apnea, and or hypotension. The middle cranial fossa floor, cavernous sinus wall dura is supplied by V1 division of trigeminal nerve.15 Thus, traction on the third nerve schwannoma resulted in the cardiovascular instability witnessed during surgery in this case.
Conclusion
Purely cystic oculomotor schwannoma is very rare and should be kept as a differential diagnosis while managing cisternal lesions and can present without third nerve palsy. Complete removal is usually not possible without a clinical deficit.
|
Study |
Characteristics of lesion |
Preoperative presentation |
Excision done |
|---|---|---|---|
|
Suenaga et al5 |
Cystic occulomotor schwannoma |
3rd nerve paresis |
Subtotal decompression |
|
Marutirao et al4 |
Cistern all occulomotor schwannoma |
3rd nerve palsy with signs of increased intracranial pressure |
Gross total decompression |
|
Asaoka et al9 |
Cisternal oculomotor schwannoma |
Chronic headache |
Gross total decompression |
|
Current study |
Cystic cisternal oculomotor schwannoma |
One episode of seizure |
Subtotal decompression |
Conflict of Interest
None declared.
Funding None.
References
- Large oculomotor nerve schwannoma–rare entity: a case report with review of literature. J Cancer Res Ther. 2014;10(4):1098-1100.
- [Google Scholar]
- Oculomotor nerve schwannoma: a case report. Brain Tumor Res Treat. 2014;2(1):43-47.
- [Google Scholar]
- Rare cranial nerve schwannomas: a retrospective review of nontrigeminal, nonvestibular cranial nerve schwannomas. J Neurosci Rural Pract. 2018;9(2):258-263.
- [Google Scholar]
- Sporadic cisternal oculomotor nerve schwannoma: a rare case with review of literature. Asian J Neurosurg. 2018;13(4):1269-1272.
- [Google Scholar]
- Oculomotor nerve schwannoma presenting as an entirely cystic homogeneous mass on magnetic resonance imaging: case report. Austin Neurosurg Open Access.. 2014;1(3):1013.
- [Google Scholar]
- Management of oculomotor nerve schwannomas in two different locations: surgical nuances and comprehensive review. Neurosurg Rev. 2012;35(1):27-34.
- [Google Scholar]
- Schwannoma in the crural cistern removed without permanent functional deficits–case report. Neurol Med Chir (Tokyo). 2003;43(2):95-99.
- [Google Scholar]
- Schwannoma of the oculomotor nerve: a case report with consideration of the surgical treatment. Neurosurgery. 1999;45(3):630-633.
- [Google Scholar]
- Asymptomatic schwannoma of the oculomotor nerve: case report. J Clin Neurosci. 2000;7(5):458-460.
- [Google Scholar]
- Oculomotor nerve schwannoma located in the oculomotor cistern. Surg Neurol. 2007;67(1):83-88.
- [Google Scholar]
- Gamma Knife surgery for schwannomas originating from cranial nerves III, IV, and VI. J Neurosurg. 2008;109:149-153. (Suppl):
- [Google Scholar]
- Complete removal of an oculomotor nerve neurinoma without permanent functional deficit. Case report. Ger J Ophthalmol. 1993;2:228-233. (4–5):
- [Google Scholar]
- Trigeminocardiac reflex: a unique case of recurrent asystole during bilateral trigeminal sensory root rhizotomy. J Cran Maxillofac Surg. 2002;30:108-111.
- [Google Scholar]
- Macroscopic innervation of the dura mater covering the middle cranial fossa in humans correlated to neurovascular headache. Front Neuroanat 2017 (e-Pub ahead of print)
- [CrossRef] [Google Scholar]







