Translate this page into:
Isolated intramedullary spinal cord cysticercosis
Address for correspondence: Dr. Zeeshan Qazi, Department of Neurosurgery, King George's Medical University, Lucknow 226 003, Uttar Pradesh, India. E-mail: tusharpatilgmc42@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neurocysticercosis is a major cause of epilepsy in developing countries. Cysticercal involvement of the spinal cord is rare even in endemic areas and accounts for 0.7 to 5.85% of all cases. We present a 19-year-old man who presented with weakness of both lower limbs and urinary complaints in the form of straining of micturition with increased frequency, in whom preoperative MRI revealed a well-defined cystic lesion in dorso-lumber cord extending from D11 to L1 level, which on pathological examination was found to be intramedullary cysticercosis.
Keywords
Cysticercosis
cystic lesion
intramedullary
neurocysticercosis
Taenia solium
Introduction
Cysticercosis is the disease caused by larval form of the tapeworm Taenia solium. Cysticercosis is the most common parasitic disease of the central nervous system.[1] Neurocysticercosis is a major cause of epilepsy in developing countries. The disease is endemic in Mexico, Central and South America, and parts of Africa and Asia, particularly Indian subcontinent, and is responsible for more than 50,000 deaths annually in these regions of the world.[23] Cysticercal involvement of the spinal cord is rare even in endemic areas and accounts for 0.7 to 5.85% of all cases.[4] Its prevalence may be underestimated, since brain cysticercosis which is more common condition, frequently occurs concomitantly. We present a 19-year-old man who presented with weakness of both lower limbs and urinary complaints in the form of straining of micturition with increased frequency, in whom preoperative MRI revealed a well-defined cystic lesion in dorso-lumber cord extending from D11 to L1 level, which on pathological examination was found to be intramedullary cysticercosis.
Case Report
A 19-year-old man was admitted in Neurosurgery department of our institute, complaining of insidious onset, gradually progressive weakness of both lower limbs for 6 months. The weakness initially started in right lower limb followed by involvement of left lower limb within a month. He also complained of increased urinary frequency for last 5 months. He had no history of fever, low backache or radicular pain. He did not complain of weakness in upper limbs. The patient was resident of Uttar Pradesh in India, which is an endemic region for neurocysticercosis. Clinical examination revealed a young man with normal cranial nerve evaluation and intact higher mental functions. Examination of lower extremities showed increased tone with medical research council (MRC) power 4/5 at all major joints. Deep tendon reflexes were brisk with bilateral positive Babinski's sign. On sensory examination, there was decreased sensation for pain and touch in both lower extremities below L2-L3 level. Joint position and vibration senses were relatively preserved. Examination of upper extremities did not reveal any abnormality.
A clinical diagnosis of myelopathy was made and magnetic resonance imaging (MRI) of thoracolumbar spine was performed [Figure 1], which revealed an intramedullary mass in the spinal cord at D11-L1 level, causing local bulging of spinal cord with mass effect. The lesion was hypointense in T1-weighted images and brightly hyperintense in T2-weighted images, with mild peripheral contrast enhancement. The patient was subjected to surgery, in which D11-L1 laminectomy with excision of cystic structure was done after myelotomy. Per-operatively, dural bulging was noted, the cyst was grayish white, soft and intramedullary in location and having well-defined plane with surrounding cord tissue. Entire cystic mass was removed [Figure 2]. The histopathology of the mass was consistent with a cysticercus cyst. The patient recovered well from surgery and was ambulating with decreased tone and sensory improvement in both extremities.

- (a) Sagittal T2-weighted magnetic resonance imaging (MRI) showing a hyperintense intramedullary cystic lesion extending from D11 to L1 vertebral levels. (b) The lesion shows mild peripheral contrast enhancement in a gadolinium-enhanced T1-weighted image. (c) The lesion is hyperintense in a coronal short tau inversion recovery (STIR) image. (d) Axial T2-weighted MRI at D12 vertebral level demonstrating the hyperintense cystic lesion
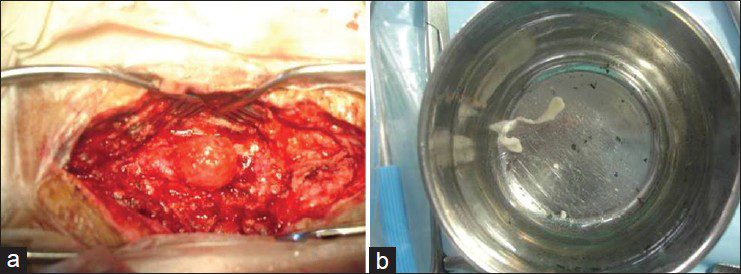
- (a) Intraoperative picture showing the intramedullary cystic lesion. (b) The excised, collapsed cyst
Discussion
Spinal cysticercosis is an unusual form of cysticercosis with an incidence of 0.7% to 5.85% even in endemic regions.[4] This low incidence may be due to inability of the cysticerci to travel to the spinal subarachnoid space. The spinal cord involvement is presumed to occur due to spread of the eggs of Taenia solium through arterial blood to the spinal cord,[5] which leads to local development of the larval form in the spinal cord manifesting as cysticercosis. When the lesion is in vesicular stage (intact viable cyst), the clinical features occur due to mass effect, causing compression of pyramidal, sensory and autonomic fibers. However, as the cyst degenerates, the body mounts an immune response against cysticercal antigens that may lead to local inflammation, causing myelitis, radiculitis and local meningitis. Furthermore, the involvement of blood vessels due to an immune-mediated vasculitic process may manifest as spinal cord infarction.
The clinical features of spinal cysticercosis may be paraparesis or quadriparesis, sensory loss, autonomic dysfunction involving bowel and bladder, radicular pain and paresthesias. Clinical presentation is dictated by the location of lesions within spinal cord, number of lesions, size of lesion and presence or absence of local inflammation. Our patient presented with features of spinal cord compression, without any inflammatory features. The clinical features were indistinguishable from any other intramedullary mass like astrocytoma or tuberculoma. In the absence of previous history of neurocysticercosis, it may be clinically difficult to make a diagnosis of spinal cysticercosis preoperatively. MR imaging can help in diagnosis of these lesions on which the cysticercal cysts appear homogeneously hypointense on T1 and hyperintense on T2 with minimal surrounding edema.
Similar cases have been described previously in literature. Guedes-Corrêa et al. described a women with an intramedullary cyst of the conus medulllaris region, who presented with low backache in the absence of any other neurological features.[4] Agale et al. presented a patient with intradural cysticercosis which resulted in spinal cord compression, causing spastic paraparesis.[6] Singh et al. noted cysticercosis involving cervico-dorsal spinal cord in a young man with seizures due to cerebral neurocystiercosis.[7] Torabi et al. described a 35-year-old man with cerebral neurocysticercosis who presented with cauda equina and Brown-Sequard syndrome due to multilevel intramedullary spinal cord cysticercal lesions and cerebrospinal fluid findings of eosinophilic meningitis.[8]
There are various therapeutic options for spinal neurocysticercosis. Medical treatment can be considered in those cases with stable neurological status or diagnosed preoperatively by CSF and serum enzyme linked immunoelectric transfer blot assay for antibody. However, in patients presenting with acute or progressively deteriorating neurological state and in those where diagnosis is missed or in doubt, surgical excision is the choice of the treatment as histopathology not only confirms the diagnosis, but early surgery also provides recovery before any irreversible cord damage takes place. Our patient was managed by surgical excision of the cysticercal lesion, in accordance with the consensus guidelines for treatment of neurocysticercosis.[9] There are a few anecdotal case reports of successful medical therapy of spinal cysticercosis with albendazole and steroids.[1011] However, at present surgical therapy remains first choice on the basis of current evidence. Spinal NCC should be considered in differential diagnosis of spinal lesion, though isolated cysticercosis of spine is difficult to suspect; however, correlation of clinical findings, history of endemic areas origin and typical MRI findings can help in making early diagnosis thus obviating the delay in surgical intervention.
Conclusions
We conclude that cysticercosis involving spinal cord is an unusual manifestation of neurocysticercosis, and its diagnosis needs a high index of suspicion. This diagnosis should be considered in a patient from endemic region with a cystic spinal cord mass. Final diagnosis is possible only after surgical excision and histopathological evaluation of the specimen.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Neurocysticercosis: Regional status, epidemiology, impact and control measures in the Americas. Acta Trop. 2003;87:43-51.
- [Google Scholar]
- Taenia solium taeniosis/cysticercosis in Asia: Epidemiology, impact and issues. Acta Trop. 2003;87:53-60.
- [Google Scholar]
- Intramedullary spinal cysticercosis simulating a conus medullaris tumor: Case report. Arq Neuropsiquiatr. 2006;64:149-52.
- [Google Scholar]
- Multilevel intramedullary spinal neurocysticercosis with eosinophilic meningitis. Arch Neurol. 2004;61:770-2.
- [Google Scholar]
- Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15:747-56.
- [Google Scholar]
- Intramedullary spinal cysticercosis cured with medical therapy: Case report and review of literature. Surg Neurol. 2009;72:765-9.
- [Google Scholar]
- Isolated intramedullary spinal cysticercosis in a 10-year-old female showing dramatic response with albendazole. J Pediatr Neurosci. 2011;6:52-4.
- [Google Scholar]






