Translate this page into:
Neuroprotective effects of tetracyclines on blunt head trauma: An experimental study on rats
Address for correspondence: Dr. Ergun Daglıoglu, Department of Neurosurgery, Neurosurgery Clinics, Ankara Numune Education and Research Hospital, Ankara, Turkey. E-mail: edaglioglu@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Prevention of primary damage caused by head trauma may be avoided with protective measures and techniques which is a public health concern. Experimental and clinical studies about treatment of head trauma were all centered to prevent secondary damage caused by physiopathological changes following primary injury. Neuroprotective features of tetracyclines were the focus of several experimental studies in the last decade. In the present study we aimed to investigate the neuroprotective effects of tetracycline in an experimental model of blunt brain injury in rats.
Materials and Methods:
32 male Sprague-Dawley rats were divided into four experimental groups (n = 8). Head trauma was not performed in control group (group 1, craniectomy only). In the second group, head trauma and craniectomy were performed. Intraperitoneal saline was used in addition to trauma and craniectomy for treatment in group 3 whereas intraperitoneal tetracycline and saline were used for treatment in group 4.
Results:
When histological examinations performed by transmission electron microscopy were evaluated, injury at ultrastructural level was demonstrated to be less pronounced in tetracycline group with decreased lipid peroxidation levels.
Conclusion:
In accordance with these findings, we conclude that systemic tetracycline administration is effective in reduction of secondary brain damage and brain edema and thus it may be considered as a therapeutic option.
Keywords
Lipid peroxidation
neuroprotection
rat head trauma
secondary brain injury
tetracycline
Introduction
Traumatic brain injury usually involves an external impact targeting cranium and it is one of the leading causes of mortality and morbidity among young adults.[1] Head traumas in Turkey are usually associated with traffic accidents with an estimated mortality of 10/100.000. A thorough and detailed research focusing on head traumas is essential.[2345] The results of previous studies on neural trauma demonstrated two stages of injury defined as: Primary injury mainly linked to physical effect and secondary injury mainly associated with responses to primary effect.[678]
Trauma is well implicated as a major cause of primary brain injury. The primary effect which is beyond the scope of this paper may be managed by preventive health measures. Secondary injury may be defined as a metabolic response to primary effect and may be prevented if proper measures were not undertaken at a hospital setting.[9] Many mechanisms have been identified to contribute to secondary brain injury including rise of excitatory amino acids, generation of free oxygen radicals and arachidonic acid metabolites, high influx of Ca++ ions into the cytoplasm and development of lactic acidosis. Recently it has been shown that activation of caspase-1 and caspase-3 as well as generation of interleukin-1 was an important step for triggering apoptosis and cell death.[101112] In fact, a simultaneous radical procedure is required to prevent steps involved in mechanisms of secondary brain injury and thus to manage traumatic inflammatory process.
Tetracycline is an antimicrobial agent which has been shown to have neuroprotective effects including inhibition of caspase-1 and 3, nitric oxide synthase, matrix metalloproteinases as well as glutamate excitotoxicity.[131415] So far, tetracyclines were proven to be beneficial against neuronal injury models.[161718] The enzymatic steps described in the above paragraph are important triggers for secondary injurious process which is involved in augmentation of primary injury. In the present study, we aim to investigate the neuroprotective effects of tetracycline on rat head trauma model of blunt cerebral injury in terms of mitochondrial and axonal degeneration as well as level of lipid peroxidation.
Materials and Methods
Experimental design
The study protocol was approved by Ethic committee of Ankara Education and Research hospital. All experiments were performed at research laboratory of the same center and specimens were evaluated at Department of Histology of Hacettepe University Medical School.
32 male Sprague-Dawley rats were divided into four experimental groups as control, sham, SF and tetracycline group. Each group consists of eight rats and details of experimental protocol from each group are summarized in Table 1.
Anesthesia and experimental procedure
All rats were kept at standard conditions before and after experimental procedure. A mixture of xylazin (Rompun®, 2% solution, 60 mg/kg) and ketamine (Ketalar® , 5% solution, 10 mg/kg) was administered intraperitoneally for anesthesia. After immobilization of rats over stereotactic frame, a blunt head injury model was performed on rats of groups 2, 3 and 4 in accordance with the previous studies.[1920] Scalp was shaved and after local preparation with povidone iodine, a longitudinal incision of 2 cm was marked at the midline. Craniectomy was performed on left parietal region with a diameter 4 mm and dura was exposed for model of trauma [Figure 1a and b].
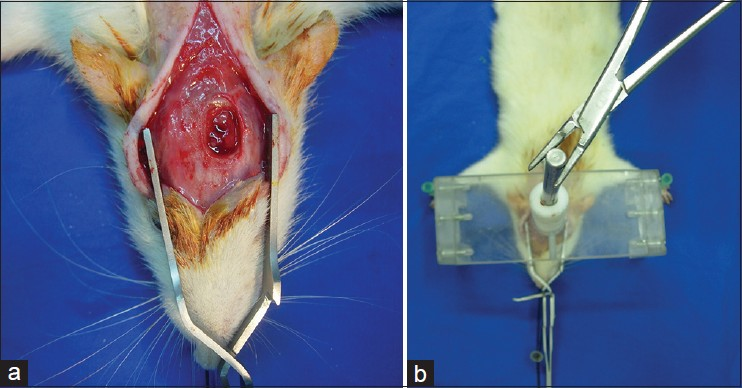
- (a and b) Surgical incision and induction of head trauma
In the present study we investigated the neuroprotective efficiency of tetracycline on brain edema secondary to brain injury following blunt head trauma. A 24 gr weighted steel rod was dropped through a cylinder (attached to a plastic plate) from 10 cm above to the craniectomy region for blunt head trauma. Experimental site was closed and tetracycline hydrochloride was administered intraperitoneally at a dose of 90 mg/kg. Tetracycline was used at the same intraperitoneal dose as it was used in other studies (90 mg/kg).[1321] The rats were kept in cages at a room temperature of 24°C under standard animal care. At 24 hours, decapitation was completed after perfusion procedures and anesthetic overdose was given to rats for sacrifice with resection of whole cerebrum as single specimen [Figure 2]. Removed brain tissue was evaluated with quantitative lipid peroxidation measurements. Qualitative histological parameters which were converted to numerical data using USS system were developed from ultrastructural evaluation.
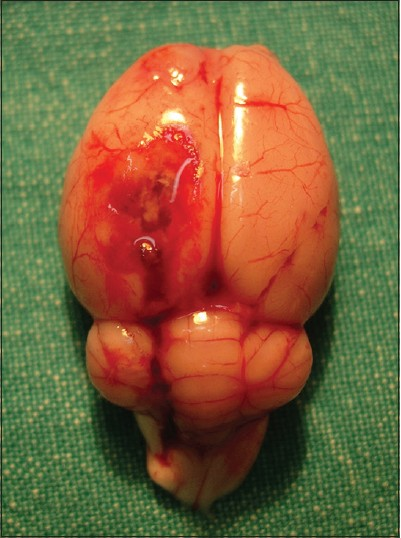
- Resected whole brain specimen after perfusion procedures and decapitation
Measurement of lipid peroxidation
After homogenization of brain tissues a solution was prepared. The solution containing 10% brain tissue within 100NMKPO4 buffer (pH 7.3). Assessment of lipid peroxidation level was performed according to Uchiyama's thiobarbituric acid method.[20] At least two graphical results obtained for each sample to determine lipid peroxidation levels as gram tissue/nanomol.[22]
Ultrastructural examination with transmission electron microscopy
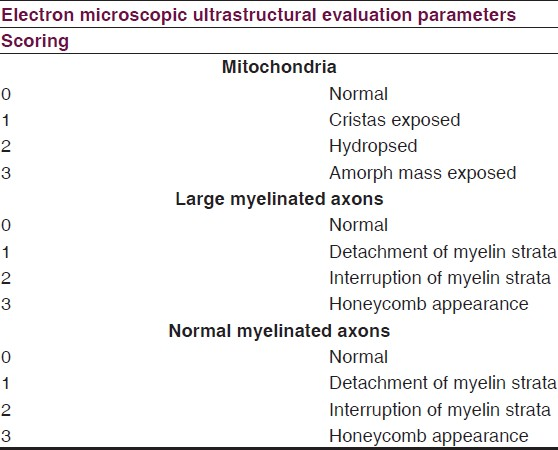
Brain tissue samples were fixed with 2.5% glutaraldehyde for 24 hours. The tissues were rinsed with Sorenson's phosphate buffer, postfixation was performed with 1% osmium tetroxide. Following serial steps epoxy resin embedded sections were prepared TEM examination. The scores obtained from TEM examination were shown in accordance with ultrastructural parameters [Table 2].
Statistical analysis
Mann Whitney-U test was performed for group comparisons and P < 0.05 was accepted as significant.
Results
Lipid peroxidation levels
Results of lipid peroxidation levels (nmol/g tissue) are shown in Table 3. The values are demonstrated as bar graphics in Graphic 1.
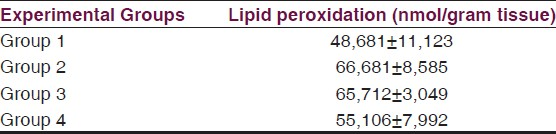
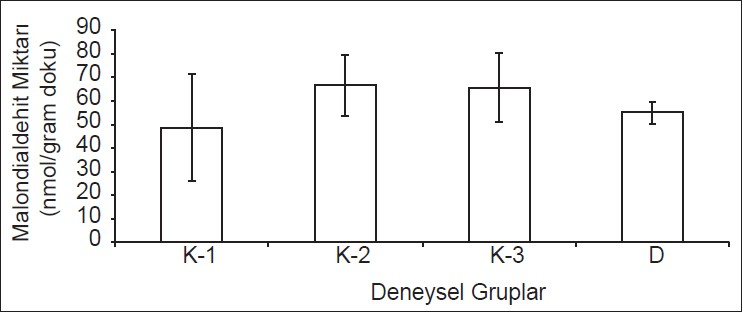
- Lipid peroxidation measurements (MDA levels) in all experimental groups (MDA levels K1: Control, K2: Trauma, K3: Saline, D: Tetracycline)
There was no significant difference between group 2 and 3 in terms of lipid peroxidation levels (P > 0.05). However, a comparison of the values of the tetracycline group (group 4) between the groups (Groups 2 and 3) designated above showed a statistical significance which proved tetracycline to be beneficial (P < 0.05).
Histological results
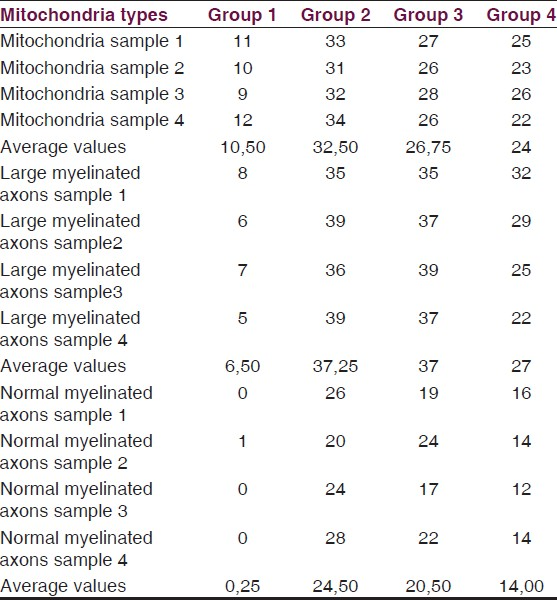
Transmission electron microscopic examination of samples and results of ultrastructural scoring system with averages for each group are demonstrated in Table 4. From each group four different samples were examined by blind investigators to reduce counting errors in each group. TEM images for each group are demonstrated in Figure 3a–d.
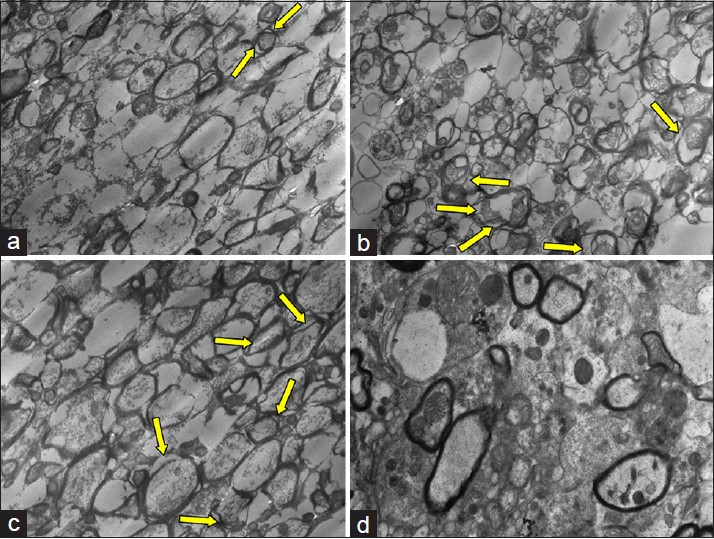
- (a) Transitional electron microscopic (TEM) image of group 1 showed minimal detachment of myelin strata, arrow shows mitochondria. (b) Detachment of myelin strata (multiple arrows). (c) Similar ultrastructural alterations with prominent detachment of myelin strata (arrows). (d) Minimal ultrastructural alteration compared to findings of groups 2 and 3
Efficacy of trauma was compared between C1 and C2 groups. A significant difference has been detected in terms of mitochondrial and axonal injury levels for these groups (P > 0.05). The systemic saline administrated group C3 and the control group C2 exhibited severe injury patterns such as myelin detachments and mitochondrial hydrops. The experimental group which received tetracycline exhibited minimal neuronal injury patterns. While intracellular vacuoles and mitochondrial hydrops were detectable in this group they were not as severe as encountered in C2 and C3 groups. The difference was significant favoring a neuroprotection for experimental samples (P < 0.05).
Discussion
Tetracycline is a well-known antibiotic proved to have protective role in neural injury. In the present study, we investigate its neuroprotective role in experimental head trauma. We found that tetracycline reduced the lipid peroxidation levels and findings on electron microscopy in experimental head trauma.
52,000 yearly deaths and 80,000 permanent severe cerebral injury are reported in the USA per year.[23] Despite the limited number of statistical studies about the frequency of head traumas in Turkey, traffic accidents are reported to be the leading cause of head trauma in Turkey. 60% of deaths due to head trauma originated from traffic accidents and a health problem which has a high mortality and morbidity.[2324]
When the head is subjected to a severe trauma, a direct damage (primary tissue damage) to cortical tissue is noted. Several mechanisms are involved in primary brain injury such as direct trauma to brain parenchyma, smashing of brain tissue by bony protuberances and penetration of brain tissue by bone fragments or foreign bodies. Skull fractures, intracranial bleeding, coup and countercoup lesion, concussion and diffuse axonal injury are the consequences of primary lesion and secondary tissue damage usually follows the primary.[7] Prevention of primary damage caused by head trauma may only be accomplished by preventive measures and is a subject of public health concern.[25]
Intracranial pressure rises after head injury which might interfere with critical level of cerebral blood flow. This reduction in blood flow further exacerbates the secondary injury process.[6] Secondary brain injury involves several mechanisms mainly associated with metabolic responses and effects of mechanical pressure.[26] Current scope of experimental and clinical studies about treatment of head trauma were to prevent secondary damage caused by pathophysiological cascades following primary injury.[78] In the present study we aimed to prevent the secondary injury in experimental head trauma.
Increase in excitatory amino acids (EAA) exists as an important mechanism after head injury and is the major concern of several studies.[2728] Free radical formation is triggered by excitatory amino acids and lipid peroxidation are accepted as the most important mechanisms that cause secondary brain damage.[829]
Despite tremendous number of experimental and clinical trials so far, a considerable and cost-effective pharmacological agent to prevent secondary brain injury was not able to be found. A substantial increase in intracranial pressure and development of hypoxia at the early stages of trauma is directly proportional with a rise in mortality and morbidity.[30] Hyperglycemia and hypoglycemia were also reported to aggravate secondary brain damage.[3132]
Tetracycline is a well-known antibiotic and its neuroprotective role was proven in several animal models within the last decade.[132133343536] Tetracyclines can easily cross blood-brain barrier due its hydrophilic properties. Its mechanism is associated with several pathways that results in reduction of apoptosis, neuroinflammation, infarct size, and vascular injury.[3537] Koistinaho, et al. reported tetracyclines to be a neuroprotective agent for brain ischemia in the rats as well as cerebral ischemia, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and multiple sclerosis.[333536] The study performed by Kraus, et al. points to protective role of minocycline on neuronal cultures.[38] In the present study we investigate the use of tetracycline on rat head trauma. A comparison of the trauma group and the tetracycline-treated group demonstrated a neuroprotective effect of drug on head trauma.
In the present study a comparison of lipid peroxidation levels between head trauma and tetracycline group did not reveal any significance designating an overt neuroprotective role of tetracycline in head trauma. Furthermore, electron microscopic examinations showed less prominent findings in the experimental head trauma group.
Conclusion
Tetracycline was shown to be beneficial in blunt head trauma when lipid peroxidation and histology of the present study was considered. We conclude that systemic tetracycline may be a considerable therapeutic option since its administration may ameliorate findings of secondary brain damage and edema.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Traumatic Cranaial Injury. In: Saveren M, Altınors N, Baykaner K, Sekerci Z, Ozyurt E, Caner H, eds. Basic Neurosurgery. Ankara: Turkish Neuorsurgery Society; 1997. p. :1-9.
- [Google Scholar]
- Oregon head and spinal cord injury prevention program and evaluation. Neurosurgery. 1989;24:453-8.
- [Google Scholar]
- Severe closed head injury in children. In: Batjer HH, Loftus CM, eds. Textbook of Neurological surgery. Baltimore, USA: Lippincott Williams and Wilkins; 2002. p. :1072-8.
- [Google Scholar]
- Handbook of Neurosurgery. New York: Thieme; 2001. p. :677-9.
- Pediatric injury control in 1999: Where do we go from here? Pediatrics. 1999;103:883-8.
- [Google Scholar]
- Mechanisms of primary head injury. In: Wilkins RH, Rengachary SS, eds. Neurosurgery (2nd ed). New York: McGraw-Hill; 1996. p. :2611-21.
- [Google Scholar]
- Pathophysiology of human head injury. In: Becker DP, Gudeman SK, eds. Textbook of Head Injury. Philadelphia: WB Saunders; 1989. p. :507-24.
- [Google Scholar]
- The effect of direct current field polarity on recovery after acute experimental spinal cord injury. Brain Res. 1992;579:32-42.
- [Google Scholar]
- Reduction of postraumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94:1213-8.
- [Google Scholar]
- Functional role of interleukin 1beta (ÝL-1beta) in ÝL-1beta-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717-24.
- [Google Scholar]
- The contrasting roles of ICE family proteases and interleukin-1beta in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc Natl Acad Sci U S A. 1996;93:5635-40.
- [Google Scholar]
- Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769-74.
- [Google Scholar]
- Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628-37.
- [Google Scholar]
- Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res. 2003;74:278-85.
- [Google Scholar]
- Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. J Neurosurg Spine. 2012;17(Suppl):157-229.
- [Google Scholar]
- Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: A twelve-week follow-up study. J Neurotrauma. 2010;27:911-21.
- [Google Scholar]
- Limited minocycline neuroprotection after balloon-compression spinal cord injury in the rat. Neurosci Lett. 2008;433:246-9.
- [Google Scholar]
- Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci. 2005;6:69.
- [Google Scholar]
- Responses to cortical injur: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67-77.
- [Google Scholar]
- Minocycline repurposing in critical illness: Focus on stroke. Curr Top Med Chem. 2013;13:2283-90.
- [Google Scholar]
- Determination of malonaldehyde precursors in tissues by thiobarbituric acid test. Ann Biochem. 1978;86:271-8.
- [Google Scholar]
- Quantification of primary and secondary lesions in severe head injury. Acta Neurochir Suppl (Wien). 1993;57:41-8.
- [Google Scholar]
- Therapeutic time window and dose responce of the beneficial effects of ketamine in experimental head injury. Stroke. 1994;25:1637-43.
- [Google Scholar]
- The role of calcium and cellular membrane dysfunction in experimental trauma and subarachnoid hemorrhage. J Neurosurg. 1985;62:698-703.
- [Google Scholar]
- Effect of hypoxia on traumatic brain injury in rats: Part 1. Changes in neurological function, electroencephalograms, and histopathology. Neurosurgery. 1987;20:848-53.
- [Google Scholar]
- Prevention of post-traumatic excitotoxic brain damage with NMDA antagonist drugs: A new strategy for the nineties. Acta Neurochir Suppl (Wien). 1992;55:49-55.
- [Google Scholar]
- The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462-8.
- [Google Scholar]
- Open-channel block N-methyl-D-aspartate (NMDA) responses by memantine: Theropeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427-36.
- [Google Scholar]
- Acut head injury: Assesment, management and prognosis. In: Hardy JD, ed. Crıtıcal Surgıcal Illness (2nd ed). Philadelphia: WB Saunders; 1980. p. :110-34.
- [Google Scholar]
- Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75:545-51.
- [Google Scholar]
- The effect of glucose administration on carbohydrate metabolism after head injury. J Neurosurg. 1991;74:43-50.
- [Google Scholar]
- A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapatic window. Proc Natl Acad Sci U S A. 1999;96:13496-500.
- [Google Scholar]
- Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia. 2013;61:1084-100.
- [Google Scholar]
- Tetracycline derivatives as anti-inflammatory agents and potential agents in stroke treatment. Ernst Schering. Res Found Workshop 2004:101-15.
- [Google Scholar]
- Tetracycline derivatives and ceftriaxone, a cephalosporin antibiotic, protect neurons against apoptosis induced by ionizing radiation. J Neurochem. 2001;78:1409-14.
- [Google Scholar]
- Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin Interv Aging. 2013;8:1089-95.
- [Google Scholar]
- Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819-27.
- [Google Scholar]






