Translate this page into:
Efficacy of Dexmedetomidine as an Adjuvant to Local Anesthetic Agent in Scalp Block and Scalp Infiltration to Control Postcraniotomy Pain: A Double-Blind Randomized Trial
Address for correspondence: Dr. Navneh Samagh, Department of Anaesthesia and Intensive Care, Adesh Institute of Medical Sciences and Research, Bathinda, Punjab, India. E-mail: navnehsamagh@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Scalp infiltration and scalp block are being used to manage postcraniotomy pain. Dexmedetomidine has been successfully used as an adjuvant in regional anesthesia. The study was intended to compare whether addition of dexmedetomidine prolonged the duration of analgesia as well as to compare the two techniques.
Aims:
The primary objective was to assess whether addition of dexmedetomidine to bupivacaine prolonged the duration of analgesia. The secondary objective was to compare between scalp nerve block and scalp infiltration as techniques for pain relief.
Settings and Design:
The randomized control study was conducted in a tertiary care center from November 2013 to October 2014.
Materials and Methods:
A total of 150 American Society of Anesthesiologists Physical Status I–II patients, aged 18–70 years undergoing elective craniotomy were included. Patients were randomized into three groups of 50 patients, i.e., Group BI (bupivacaine infiltration), Group BDI (bupivacaine and dexmedetomidine infiltration), and Group BDNB (bupivacaine and dexmedetomidine scalp nerve block). Patient's pain score, pain-free interval, rescue analgesic requirement, and hemodynamic and respiratory parameters were noted for 48 h. Patients were followed up at 1 and 3 months to assess postcraniotomy pain.
Results:
Pain-free period was significantly longer in Group BDNB than Groups BDI and BI (P < 0.0001) and pain control was better in dexmedetomidine containing groups than in bupivacaine group (BI) (P < 0.0001). The rescue analgesic requirement was significantly lower in Group BDNB and Group BDI compared to Group BI.
Conclusion:
The addition of dexmedetomidine (1 μg/kg) to bupivacaine prolonged the pain-free period. Scalp nerve block is a superior technique than scalp infiltration.
Keywords
Dexmedetomidine
postcraniotomy pain
scalp nerve block
INTRODUCTION
Postcraniotomy pain is a pounding or pulsating headache, moderate-to-severe in intensity seen in 60%–84% of patients within 48 h postoperative period.[12] It is predominantly superficial and somatic in origin.[3] Persistent acute pain gives rise to chronic pain.[4] Diagnosis and quantification of pain are difficult in neurosurgical patients due to their inability to express pain or altered sensorium.[5] Pain causes sympathetic stimulation, thus increasing the risk of bleeding and poor outcome.[6]
Opioids provide excellent analgesia but can interfere with neurological assessment of these patients. Respiratory depression resulting from opioids can lead to hypercarbia resulting in raised intracranial pressure (ICP).[78] Nonsteroidal anti-inflammatory drugs (NSAIDs) may increase bleeding risk while paracetamol alone is ineffective.[79] Regional analgesia techniques such as scalp infiltration and block have been practiced in neurosurgical patients and provide adequate analgesia and allow neurological assessment with a lower incidence of systemic complications.[101112] Dexmedetomidine has been successfully used as adjuvant in regional anesthesia.[13141516]
Limited literature is available on the efficacy of dexmedetomidine with local anesthetics for postoperative analgesia after craniotomy. The primary objective of this study was to assess if addition of dexmedetomidine to bupivacaine prolonged the duration of analgesia. The secondary objective was to compare between scalp nerve block and infiltration as techniques for pain relief.
MATERIALS AND METHODS
This double-blind, parallel group study was conducted in 150 patients who were randomly divided into three equal groups of 50 patients each (1:1:1). Ethical approval for this study was provided by the Institutional Ethics Committee. Written informed consent was obtained from the patients. The trial is registered at ClinicalTrials.gov, number NCT02866409. This manuscript adheres to the applicable EQUATOR guidelines.
Eligible candidates were patients aged 18–70 years, American Society of Anesthesiologists Class I and II, undergoing elective craniotomy. During preanesthesia visit, patients were made familiar with the Numerical Rating Scale (NRS) (0–10, with 0 = no pain, 10 = worst pain ever).[17] Patients with Glasgow Coma Scale (GCS) <15, not able to comprehend NRS, incision extending beyond the field of the block, chronically on analgesics or narcotic medications, or patients planned for postoperative mechanical ventilation were not included in the study. Patients with postoperative GCS < 15, poor cognitive function, having fever (≥39°C), or require mechanical ventilation in the postoperative period were excluded from the study.
We conducted a pilot study in 10 patients undergoing craniotomy to observe severity of pain in the postoperative period who received bupivacaine infiltration at site of skin incision at the end of surgery. Assuming mean difference of 2 points in NRS with standard deviation (SD) of 2.5 (from pilot study), sample size in our study came out to be 47 subjects per group at a power of 90% and confidence interval of 95%. Expecting attrition, it was decided to include 50 patients in each group with a total of 150 patients in the study.
The 150 patients included in the study were randomly allocated into three equal groups (50 each) by computer-generated random numbers. If any patient was excluded in the postoperative period, the same random number code was allocated to the next patient to ensure an equal sample size without any modification in the original random number table. The random numbers were then kept in opaque-sealed envelopes, numbered sequentially. The envelopes were opened just before shifting the patient inside the operation theater and the study drug was prepared in 20 ml syringes by a person not involved in the study. The drug was administered as scalp block or infiltration after completion of surgery, and the postoperative data were collected by an investigator who was blinded to the group assigned to the patient.
A standard anesthesia technique was followed in all the patients. Injection morphine 0.1 mg/kg body weight was given as premedication. The patients were induced with propofol or thiopentone, and trachea were intubated following administration of vecuronium 0.1 mg/kg. Anesthesia was maintained with intravenous or inhalational anesthetic agent along with nitrous oxide-oxygen mixture (60:40) and intermittent doses of intravenous vecuronium. Intravenous morphine was repeated at a dose of 0.05 mg/kg if duration of surgery was >3 h. Total amount of intraoperative morphine in mg/kg was noted. Patients were reversed and trachea was extubated according to standard anesthesia protocol.
After the closure of scalp incision, patients in Group BI received incision-site infiltration with bupivacaine (0.25%), Group BDI received incision-site infiltration with combination of bupivacaine (0.25%) and dexmedetomedine (1 μg/kg), and Group BDNB received scalp nerve block with bupivacaine (0.25%) and dexmedetomedine (1 μg/kg). Supraorbital, supratrochlear, zygomaticotemporal, auriculotemporal, greater auricular, and greater and lesser occipital nerves were blocked. In all three groups, 20 ml of study drug was used. In infiltration group, 1/3rd of the total volume of the drug was infiltrated in the muscle while 2/3rd of the drug was injected into the subcutaneous tissue. To prevent local anesthetic toxicity, the dose of bupivacaine was kept < 2 mg/kg in all the three groups.
Patients were shifted to the Postanesthesia Care Unit (PACU) and were assessed for hemodynamic parameters, i.e., heart rate (HR), systolic and diastolic blood pressure (SBP and DBP), respiration rate (RR), and oxygen saturation (SpO2) on arrival and then at 15 min interval for 2 h. Patients were followed up for 48 h and these parameters were recorded at 4, 8, 12, 16, 20, 24, 36, and 48 h after surgery. To check fitness for proper evaluation of NRS, patients were evaluated for cognitive function 30 min after shifting to PACU using a modified questionnaire of Short Orientation Memory Concentration (SOMC) test.[18] Subjects who were able to recall and count with minimal mistakes (1–3) were regarded as good, ≥3 mistakes were regarded as fair, and those not able to recall at all were regarded as poor cognitive function. In patients with poor cognitive function at 30 min, the same test was repeated at 1 h. If the grading was again poor, then that patient was excluded from the study.
Postoperative pain was assessed by NRS, wherein 0 meant no pain and 10 meant the worst imaginable excruciating pain.[17] It was assessed on arrival to PACU and at regular interval till 48 h after surgery. When NRS score was >4, rescue analgesic paracetamol 1 g was administered intravenously. Time of first rescue analgesic administration from the time of test drug administration was recorded as the pain-free interval. The total rescue analgesic requirement in 24 and 48 h was noted. Prolonged postcraniotomy pain was assessed using NRS by telephonic communication at 1- and 3-month interval, postoperatively.
Any adverse events such as bradycardia (HR ≤40), hypotension (SBP < 90 mm of Hg) were recorded and treated by rescue drugs: atropine 15 μg/kg and mephentermine 3 mg/dose, respectively. Total amount of rescue drug requirement in 24 and 48 h was noted. Level of sedation in the postoperative period was assessed using Modified Ramsay Sedation Scale (MRSS).[19] Respiratory depression (RR < 8 breaths/min) was recorded and managed accordingly. Postoperative nausea and vomiting (PONV) was assessed on a 4-point PONV Scale with 1 as no PONV, 2 as mild PONV (only mild nausea/one emetic episode/nausea lasting < 10 min, 3 as moderate PONV (patient has 1–2 emetic episodes/moderate to severe nausea and antiemetic therapy is required, and 4 as severe PONV wherein the patient has >2 emetic episodes/is nauseated more than twice/more than one antiemetic required to treat PONV. Rescue antiemetic ondansetron 0.15 mg/kg was given intravenously if PONV score was >2.[20] Any other complications were noted.
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Packages for the Social Sciences, Chicago, IL, USA) version 22. The continuous data were presented as mean ± SD or median and interquartile range, as appropriate. Normality of quantitative data was checked by measures of Kolmogorov–Smirnov tests of normality. For age and weight, one-way ANOVA was used for comparison of means of three groups. Hemodynamic variables HR, SBP, DBP, RR, and SpO2 were compared by ANOVA. Pain scores, modified SOMC score, and PONV score were compared by Kruskal–Wallis test followed by Mann–Whitney test for two groups. For time-related scores, Wilcoxon signed-rank test was applied. Pain-free period was skewed, so Kruskal–Wallis test was applied and Kaplan–Meier analysis was done. Rescue drugs, site of craniotomy were described as frequencies and percentages and were compared using Chi-square or Fisher's exact test whichever was applicable. All tests were two-sided. The rescue analgesic requirement was compared in the three groups by using ANOVA. The results were presented as mean with SD. Comparison between two groups was done by post hoc test. Bonferroni correction was applied in post hoc multiple comparisons. P < 0.05 was considered to indicate statistical significance.
RESULTS
In this study, 168 patients undergoing craniotomy from November 2013 to October 2014 were enrolled. Eighteen patients were excluded and 150 patients were included. Out of 18 patients who were excluded, eight patients required postoperative mechanical ventilation, six patients had GCS score < 15 and were not extubated, and four patients had poor cognitive function. The random number code given to these patients was allocated to the next patient to ensure an equal sample size without any modification to the original random number table. A total of 150 patients were randomly allocated into three groups with 50 patients in each group: Group BI, Group BDI, and Group BDNB.
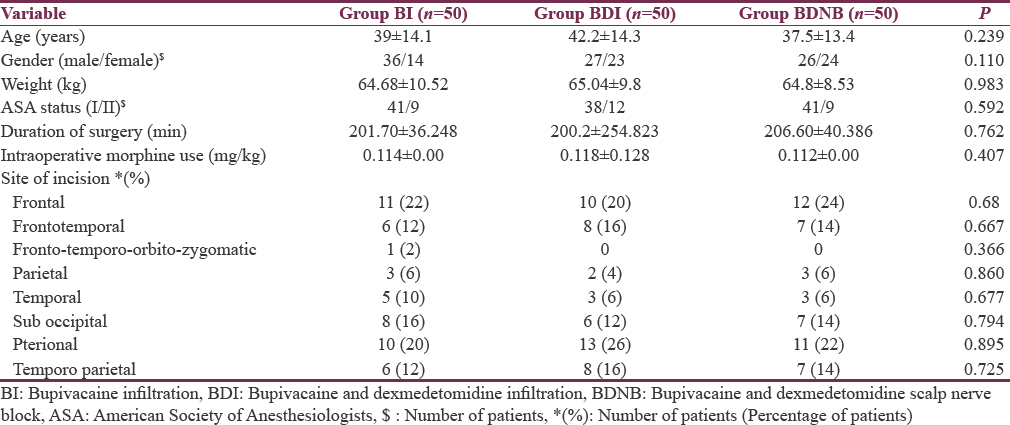
The demographic characteristics, surgical site, duration of surgery, and intraoperative analgesic (morphine) consumption were comparable between the groups [Table 1].
The postoperative cognitive function (SOMC) at 30 min was good/fair in 39/11 patients in BI group, 37/13 patients in BDI group, and 36/14 patients in BDNB group (P = 0.967). Hence, no patient enrolled in the study was excluded from the study for poor cognition and inability to carry out NRS scoring.
In postoperative 48 h, HR, SBP, DBP, SpO2, and RR were comparable in all the three groups at different time periods. No patient in any of the group developed respiratory depression or desaturation during study period.
Pain-free period was longer in patients receiving dexmedetomidine, i.e., Group BDNB and BDI. It was 12 (8–16) h in Group BDNB compared to 8 (8–12) h in Group BDI and 4 (4–8) h for Group BI (P < 0.0001) [Table 2 and Figures 1, 2]. The difference in rescue analgesic requirement was found to be statistically significant at both 24 and 48 h (P < 0.0001 and P < 0.0001, respectively) in three groups. The total rescue analgesic drug (injection paracetamol) requirement was significantly less in BDNB group and BDI group as compared to BI group (BI > BDI > BDNB) (P ≤ 0.0001) [Figure 3]. The total rescue analgesic requirement was decreased by 56% versus 23% in 24 h and 54% versus 19% in 48 h in Group BDNB and Group BDI, respectively, when compared to the Group BI (P < 0.001). Pain scores were compared at different time points after the surgery till 48 h. There was significant difference in the NRS for pain in patients in three groups after surgery (P < 0.05). No patient experienced pain till 2 h of receiving block or infiltration in any of the group [Figure 4].

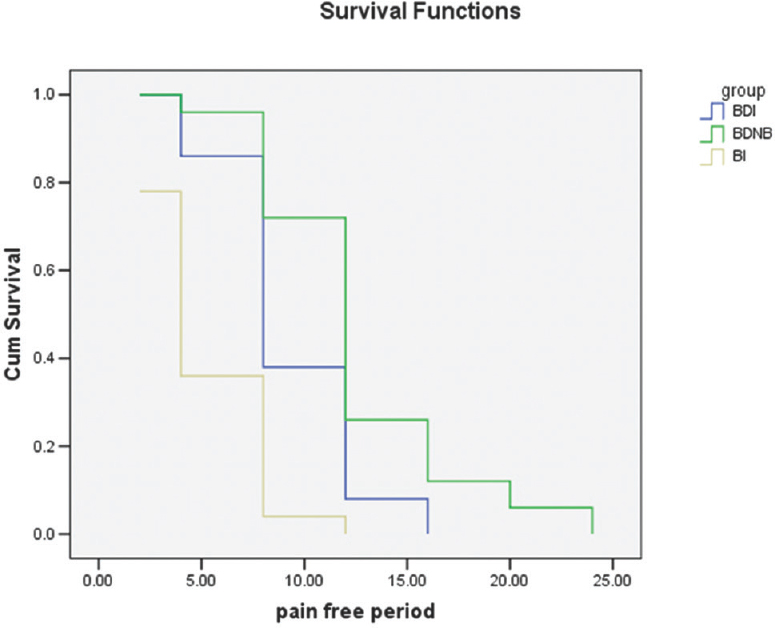
- Comparison of pain-free period – Kaplan–Meier survival graph
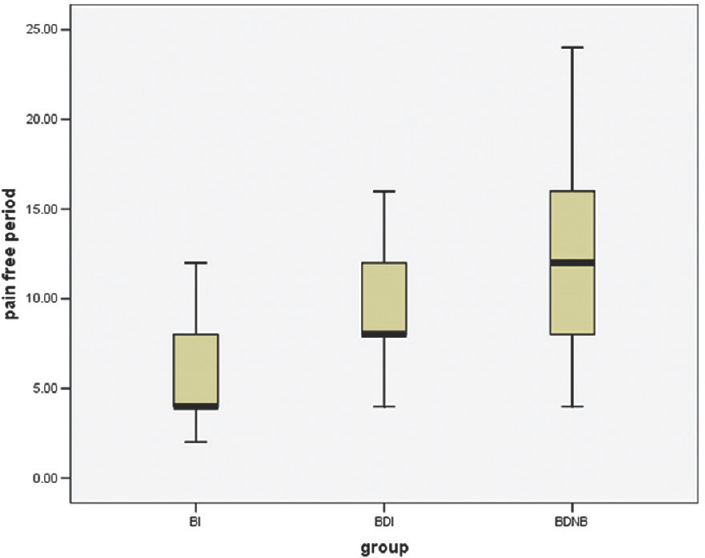
- Comparison of pain-free period among the groups
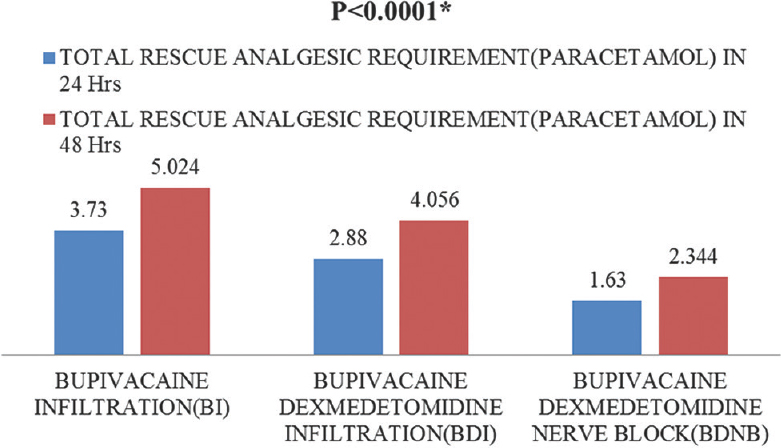
- Comparison of total rescue analgesic requirement (paracetamol in g) in 24 and 48 h
![Comparison of pain scores between the groups using box plot (data presented as median [interquartile range])](/content/150/2018/9/1/img/JNRP-9-73-g006.png)
- Comparison of pain scores between the groups using box plot (data presented as median [interquartile range])
The postoperative sedation score was higher in Groups BDNB and BDI compared to Group BI till 120 min from the time of administration of the study drug (P < 0.001). However, none of the patients in the BDNB and BDI groups had sedation score >3, i.e., all the patients were arousable with verbal stimulus. Incidence of PONV was comparable among the three groups till 48 h in the postoperative period.
All patients were interviewed telephonically at 1 month and 3 months. The incidence of prolonged pain observed at 1 month in BDNB, BDI, and BI groups was 14%, 30%, and 40%, respectively, the difference was statistically significant (P = 0.014), while the incidence of chronic pain at 3 months in BDNB, BDI, and BI groups was 4%, 10%, and 12%, respectively, and was statistically insignificant (P = 0.156) [Table 2].
DISCUSSION
In our study, we found that the addition of dexmedetomidine to bupivacaine in scalp infiltration (BDI) as well as scalp nerve block (BDNB) significantly prolonged the pain-free interval when compared to bupivacaine infiltration group (BI) (BDNB and BDI >BI, P < 0.001). The pain scores were significantly lower in the nerve block (BDNB) group as compared to the scalp infiltration groups (BI and BDI) (P < 0.05). We also observed that the pain scores were lower in patients who received dexmedetomidine with bupivacaine (BDI and BDNB) than the patients who received bupivacaine alone (BI) [Figure 4]. We observed that the rescue analgesic requirement was significantly lower in Group BDNB patients as compared to Group BDI and Group BI patients (BDNB vs. BI/BDI P < 0.001). When dexmedetomidine was added to the local anesthetic agent, total rescue analgesic requirement was decreased by 56% versus 23% (24 h) and 54% versus 19% (48 h) in Group BDNB and Group BDI, respectively, when compared to the Group BI (P < 0.001). As the interventions were implemented in adults of both sexes, and in craniotomies involving all types of incisions, this could benefit all the patients undergoing craniotomies.
Regional anesthesia techniques in the form of scalp block and scalp infiltration block the nerves that supply sensation to the scalp and provide good pain control with lesser systemic adverse effects. The addition of dexmedetomidine to a local anesthetic agent for surgical-site infiltration or peripheral nerve block has been found to prolong the duration of analgesia in various studies.[2122] Dexmedetomidine has been found to block the hyperpolarization-activated cation current (Ih current) which is activated by the initial hyperpolarization of the nerve. The Ih current is known to bring the nerve back to the normal resting potential state, and hence, its blocking by dexmedetomidine results in prolonged hyperpolarization of the nerve. The cited effect of dexmedetomidine upon the Ih current appears to be more prominent in C fibers (pain) than in A alpha fibers (motor), and therefore, the predominant sensory block can be observed.[23] The scalp is richly innervated by C fibers and this may explain the prolongation of duration of analgesia in the BDNB and BDI groups where dexmedetomidine was added to local anesthetic agent bupivacaine.[5]
In a study conducted by Bala et al.,[24] patients who received scalp block with 0.5% bupivacaine and epinephrine were found to be pain-free up to 4 h in the postoperative period. Median pain scores were significantly lower in bupivacaine group for up to 6 h as compared to placebo (P < 0.01). The median duration for the requirement of the first dose of diclofenac was significantly longer (P < 0.01) and the number of doses was significantly less (P < 0.01) in the bupivacaine group as compared to the placebo.[24]
Scalp infiltration with local anesthetic agent is a preferred and commonly used technique by the surgeons. Bloomfield et al.[11] in their study utilized preoperative bupivacaine for scalp infiltration and found blunting of intraoperative hemodynamic responses, reduced postoperative pain, but had no effect on the postoperative hemodynamic. Batoz et al. in their study used 20 mL of 0.75% ropivacaine for surgical-site infiltration at the end of the surgery and evaluated acute pain using visual analog scale (VAS) scores hourly for first 24 h along with the total consumption of rescue analgesic (injection nalbuphine). The author found VAS scores were significantly lower and nalbuphine consumption was nonsignificantly lower in the ropivacaine group during first 24 h after surgery (P = 0.054).[25] In our study, the patients who received scalp nerve block had a significantly longer median pain-free interval of 12 h as compared to patients who received scalp infiltration with and without dexmedetomidine as 8 and 4 h, respectively (P < 0.0001).
Kang in a study comparing ropivacaine dexmedetomidine infiltration to ropivacaine only infiltration group in post inguinal herniorrhaphy patients, found significantly lower pain scores (VAS) until 24 h with lesser fentanyl consumption until 12 h postoperatively in the ropivacaine dexmedetomidine group.[22]
Inadequately treated acute pain may increase the incidence of chronic pain.[26] We followed our patients at 1 month and 3 months for the presence of headache. Significantly lesser number of patients complained of headache in Group BDNB as compared to Group BDI and Group BI patients (n = 7, 15, 20) at 1 month (P = 0.014) which were lesser but nonsignificant at 3 months (n = 2, 5, 6) as in the study by Batoz et al.[25] In our patients, early pain was controlled well with the study drug infiltration or nerve block and adequate analgesia was ensured with rescue analgesic to keep pain score always < 4 which leads to the lower incidence of prolonged pain in our patients.
Hypotension and bradycardia are considered to be the most prominent adverse effects of α-2 agonists.[27] However, in this study, none of the patients developed bradycardia and hypotension as we used a lower dose of dexmedetomidine (1 μg/kg). In this study, the sedation scores were higher in patients receiving dexmedetomidine with bupivacaine (Group BDI and Group BDNB) than the patients who received bupivacaine alone (Group BI) for first 2 h (P < 0.05), but MRSS was always ≤3; hence, no patient had airway compromise due to excessive sedation and did not desaturate during the study period. Dexmedetomidine has been known to produce arousable sedation, i.e., the patient is sedated but responds to verbal instructions.[28] Incidence of PONV was lower in our study as compared to previous studies.[29] It may be due to better pain control in the patients. None of our patients developed complications such as respiratory depression, hypoxemia, vascular puncture, scalp infection, or hematoma during the follow-up period.
Other strategies of analgesia including opioid derivatives such as tramadol, codeine, and morphine have been used in neurosurgical patients. However, these agents are being avoided for a long time owing to their potential side effects such as sedation, respiratory depression, nausea, vomiting, and miosis which may mask the signs of an adverse intracranial event.[3031] Respiratory depression resulting from opioids can lead to hypercarbia resulting in raised ICP.[78] Other options comprise of nonopioid analgesics such as NSAIDs and paracetamol. NSAIDs can increase the risk of bleeding as well as postoperative hematoma formation due to their antiplatelet function which could be fatal in neurosurgical patients.[3233] However, diclofenac if given preoperatively as a preemptive analgesic has been found to decrease the incidence as well as the severity of postcraniotomy headache in one of the studies.[34] Paracetamol alone provides inadequate analgesia so must be used as a part of multimodal analgesic therapy.[79]
We did not have the record of preoperative headache in our patient population. Patients with headache in the preoperative period may have more analgesic requirement due to preoperative sensitization. This may be a limitation of our study. Second, we have not divided cases on the basis of supra- or infra-tentorial craniotomies. However, most of the cases included in this study were supratentorial in origin (86%). This may be another limitation of this study.
CONCLUSION
We conclude that scalp nerve block using dexmedetomidine as an adjuvant to bupivacaine may be considered as one of the method of choice for managing postcraniotomy pain. It is safe, efficacious, easy to administer, less time-consuming with very few side effects. Dexmedetomidine when added to bupivacaine as an adjuvant prolongs the duration of pain relief after craniotomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Acute and chronic pain following craniotomy: A review. Anaesthesia. 2005;60:693-704.
- [Google Scholar]
- Anatomic and physiologic basis of nociception and pain. In: Bonica JJ, ed. The Management of Pain (2nd ed). Philadelphia: Lea & Febiger; 1990. p. :28-94.
- [Google Scholar]
- Postoperative pain in neurosurgery: A pilot study in brain surgery. Neurosurgery. 1996;38:466-9.
- [Google Scholar]
- Pain following craniotomy: Reassessment of the available options. Biomed Res Int 2015 2015:509164.
- [Google Scholar]
- Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93:48-54.
- [Google Scholar]
- Postoperative hematoma: A 5-year survey and identification of avoidable risk factors. Neurosurgery. 1994;35:1061-4.
- [Google Scholar]
- Analgesia after intracranial surgery: A double-blind, prospective comparison of codeine and tramadol. Br J Anaesth. 1999;83:245-9.
- [Google Scholar]
- Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14:96-101.
- [Google Scholar]
- Preincision 0.25% bupivacaine scalp infiltration and postcraniotomy pain: A randomized double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2003;15:234-9.
- [Google Scholar]
- The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg. 1998;87:579-82.
- [Google Scholar]
- Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93:1272-6.
- [Google Scholar]
- The analgesic effect of intrathecal dexmedetomidine or clonidine, with bupivacaine, in trauma patients undergoing lower limb surgery: A randomised, double-blind study. Anaesth Intensive Care. 2013;41:51-6.
- [Google Scholar]
- Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280-4.
- [Google Scholar]
- Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: A systematic review and meta-analysis. Br J Anaesth. 2013;110:915-25.
- [Google Scholar]
- Comparison between clonidine and epinephrine admixture to lidocaine in brachial plexus block. Anesth Analg. 1992;75:69-74.
- [Google Scholar]
- Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22:1453-8.
- [Google Scholar]
- Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734-9.
- [Google Scholar]
- Metoclopramide versus ondansetron in prophylaxis of nausea and vomiting for laparoscopic cholecystectomy. Am J Surg. 2001;181:138-41.
- [Google Scholar]
- Evaluation of the analgesic efficacy of local dexmedetomidine application. Clin J Pain. 2011;27:377-82.
- [Google Scholar]
- The effect of dexmedetomidine added to preemptive ropivacaine infiltration on post-operative pain after inguinal herniorrhaphy: A prospective, randomized, double-blind, placebo-controlled study. Eur Surg. 2012;44:274-80.
- [Google Scholar]
- Alpha-2 adrenoceptor agonists as adjuncts to peripheral nerve blocks in children – Is there a mechanism of action and should we use them? Paediatr Anaesth. 2012;22:421-4.
- [Google Scholar]
- Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care. 2006;34:224-7.
- [Google Scholar]
- The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109:240-4.
- [Google Scholar]
- Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618-25.
- [Google Scholar]
- Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548-51.
- [Google Scholar]
- Adding clonidine or dexmedetomidine to lidocaine during Biers block: A comparative study. J Med Sci. 2008;8:660-4.
- [Google Scholar]
- Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: A randomized, double-blind, and controlled study. World Neurosurg. 2015;83:108-13.
- [Google Scholar]
- Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106:210-6.
- [Google Scholar]
- A survey of post-craniotomy analgesia in British neurosurgical centres: Time for perceptions and prescribing to change? Br J Neurosurg. 2009;23:538-42.
- [Google Scholar]
- ‘Scheduled’ dosing of lornoxicam provides analgesia superior to that provided by ‘on request’ dosing following craniotomy. Eur J Anaesthesiol. 2009;26:633-7.
- [Google Scholar]
- A single preoperative dose of diclofenac reduces the intensity of acute postcraniotomy headache and decreases analgesic requirements over five postoperative days in adults: A single center, randomized, blinded trial. J Neurol Sci. 2015;353:70-3.
- [Google Scholar]






