Translate this page into:
An Entropy-Based Prospective Randomized Controlled Trial to Evaluate the Analgesic and Hypnotic Effects of Equipotent Doses of Sevoflurane and Isoflurane in Patients Presenting for Spine Surgeries
Ajay Prasad Hrishi, MD, DM, EDAIC Division of Neuroanesthesia, Department of Anesthesiology, Sree Chitra Tirunal Institute for Medical Sciences and Technology Thiruvananthapuram, Kerala India drajay@sctimst.ac.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives Minimal alveolar concentration (MAC) of anesthetic agents has been considered a suitable measure of the potency of inhalational anesthetics. Furthermore, it is assumed that equi-MAC concentrations of different anesthetic agents have a similar potency in suppressing responses to painful stimuli. Isoflurane and sevoflurane are two commonly used volatile anesthetic agents in spine surgeries. Therefore, these agents' hypnotic and analgesic potencies should be distinguished and comprehended for the optimal administration of anesthesia. Consequently, we undertook this study to compare the analgesic and hypnotic potencies between these agents at equi-MAC concentrations, using the entropy monitor.
Materials and Methods Forty patients undergoing lumbar spine surgery were randomly assigned to two groups receiving either isoflurane (n = 20) or sevoflurane (n = 20). After induction, maintenance of anesthesia was done with age-corrected 1.0 MAC of either isoflurane or sevoflurane. A standardized noxious stimulus was provided to all the patients after achieving a steady state of 1.0 MAC. The state entropy (SE), response entropy (RE), and RE–SE were recorded at baseline, prestimulus, and poststimulus time points in both groups.
Statistical Analyses Data are presented as frequency and percentages for categorical variables and mean ± standard deviation for continuous variables. The comparison of categorical variables between the two groups was made using the Fisher's exact test, and the Student's t-test was used for continuous variables. A p-value of < 0.05 was considered to be statistically significant.
Results At age-corrected 1.0 MAC, there was no significant difference in the SE, RE, and RE–SE in both the groups at any time point.
Conclusion Our study shows that during a steady state of age-corrected 1.0 MAC single-agent anesthesia, sevoflurane and isoflurane have comparable analgesic and hypnotic potencies as measured by entropy indices when a standardized nociceptive stimulus is provided.
Keywords
entropy
analgesia
isoflurane
sevoflurane
Introduction
General anesthesia consists of the four components of hypnosis, analgesia, immobility, and the maintenance of homeostasis. Therefore, monitoring the respective components would allow tailoring drug administration to facilitate balanced anesthesia when a clinician pharmacologically induces this reversible state of unconsciousness.1
Minimal alveolar concentration (MAC) of anesthetic agents has been considered a suitable measure of the potency of inhalational anesthetics. Furthermore, it is assumed that equi-MAC concentrations of different anesthetics have a similar potency in suppressing responses to painful stimuli.2 However, MAC as a measure of potency has been questioned since the suppression of mobility is mediated by spinal α-motor neuron depression and not the area of its hypnotic action, that is, the cerebral cortex.3 However, the fact that we can quantify MAC for various volatile anesthetics allows us to compare various effects of these anesthetic agents at equi-MAC concentrations (e.g., 0.5 MAC, 1.0 MAC, and 2.0 MAC).
Isoflurane and sevoflurane are two commonly used volatile anesthetic agents in spine surgeries. These agents' hypnotic and analgesic potencies should be distinguished and comprehended for the optimal administration of anesthesia. We undertook this study to compare the analgesic and hypnotic potencies between these agents using the entropy monitor. A quantitative comparison of their hypnotic and analgesic effects can help us choose the ideal inhaled anesthetic in our clinical practice.
Materials and Methods
After obtaining approval from the institutional ethics committee, a prospective randomized study was initiated. Consenting patients aged between 18 and 60 years scheduled for elective lumbar disc surgery were included in the study. Patients with American Society of Anesthesiologists physical status classification of III and higher, neurologic or psychiatric ailments, obesity and underweight patients, alcohol or drug abuse, any medications affecting the nervous system, that is, sedatives, anxiolytics, prior chronic usage of analgesics were excluded from the study. Using a web-based response, a random-permuted block randomization algorithm randomly allocated the patients into two groups (isoflurane and sevoflurane groups) of 20 each. Allocation concealment was ensured with opaque serially numbered envelopes containing protocol with the name of the agent to be used.
Premedication drugs such as anxiolytics and anticholinergics were avoided in the study population. In the operating room, standard preinduction monitoring, comprising electrocardiography, noninvasive blood pressure, and pulse oximetry (SpO2), was attached. According to the manufacturer's instructions, the entropy electrode was applied to the patient's forehead and connected to the monitor (M-Entropy module for S/5 Anesthesia Monitor, GE Healthcare). State entropy (SE) and response entropy (RE) and the difference between them (RE–SE) were surrogate measures to assess the hypnotic and analgesic levels, respectively. General anesthesia was induced with Inj. propofol 2 mg/kg and tracheal intubation was facilitated with Inj. succinylcholine 2 mg/kg. In addition, lignocaine 2 mg/kg was administered to blunt the autonomic responses to intubation. The peripheral nerve stimulator electrodes were placed over the ulnar nerve on the volar aspect of the distal forearm. A train-of-four (TOF) count of 0 was ensured prior to intubation using a neuromuscular monitor device (M-NMT MechanoSensor, GE Healthcare, Finland). Hemodynamic surges during laryngoscopy and intubation were promptly treated with a titrated dose of esmolol. After intubation, mechanical ventilation with air:O2 (1:1) mixture was initiated. Temperature and end-tidal CO2 monitoring was instituted to ensure normothermia and normocarbia.
At this juncture, the volatile anesthetic agent was introduced by overpressurization to target an age-corrected MAC of 1.0. End-tidal anesthetic concentration was continuously measured using the gas analyzer (GE Datex Ohmeda S5 Anesthesia Monitor). The noxious stimulus was provided after 20 minutes to ensure the steady-state concentration of the volatile agent and to avoid the residual effects of propofol. We also confirmed a TOF count of 4 prior to the stimulus. The noxious stimulus was provided to the subject by tetanic stimulation (square wave, 70 mA stimulus, 30-second duration at 50 Hz), and the postnoxious stimulus study parameters were obtained. Opioids were administered after the recording of the poststimulus values.
The study parameters, namely, heart rate (HR), mean arterial pressure (MAP), SE, RE, and RE–SE were recorded at three time points: before anesthetic induction, prior to providing noxious stimulus, and after application of noxious stimuli. The baseline and prenoxious stimulus values were recorded as the mean values calculated over 1 minute. The postnoxious stimulus values were the maximal readings recorded within 1 minute of the stimulus application.
During the study duration, hemodynamic derangements were promptly managed. If the entropy values were > 70, additional sedatives/analgesics would be administered, and such patients were excluded from the study.
Statistical Analysis
The primary outcome of interest was the measure of analgesia, that is, RE–SE. Since there has not been any previous study comparing analgesic properties of isoflurane and sevoflurane using entropy indices, the power analysis for sample size calculation was based on the RE–SE difference considered significant between any two anesthetic agents. Therefore, a difference of 4, derived from previous studies, was utilized.4 5 The study was designed to have a power of 90% to detect a statistical significance of 0.05 in the RE–SE difference between isoflurane and sevoflurane groups. To meet these criteria, we included 20 subjects in each group.
Statistical analyses were performed using the SPSS 17.0 version. Data are presented as frequency and percentages for categorical variables and mean ± standard deviation for continuous variables. The Shapiro–Wilk's test was used to confirm the normalcy of distribution for categorical variables. The comparison of categorical variables between the two groups was made using the Fisher's exact test, and the Student's t-test was used for continuous variables. A p-value of < 0.05 was considered to be statistically significant.
Results
Fifty-six patients presenting for spinal surgery were screened for the study. Twelve patients were ineligible based on the exclusion criterion (Fig. 1). One patient withdrew consent and was excluded from the study. Therefore, 21 subjects were included in each group. Two patients (one from each group) were excluded from the study because of the administration of fentanyl. Thus, data of 20 subjects in each group were taken for final analysis (Fig. 1). Patient baseline demographic characteristics and entropy indices were comparable between groups (Table 1).
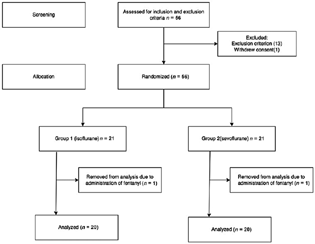
-
Fig. 1 Consort flow diagram for the recruitment and allocation of subjects in the study.
Fig. 1 Consort flow diagram for the recruitment and allocation of subjects in the study.
|
Parameters |
Group 1 (isoflurane) |
Group 2 (sevoflurane) |
p-Value |
|---|---|---|---|
|
Age (y) (mean ± SD) |
45 ± 12 |
41 ± 14 |
0.33 |
|
Male:female ratio |
12:8 |
10:10 |
0.75 |
|
Height (cm) (mean ± SD) |
159 ± 12 |
155 ± 10 |
0.26 |
|
Weight (kg) (mean ± SD) |
69 ± 15 |
73 ± 18 |
0.45 |
Abbreviation: SD, standard deviation.
Note: p < 0.05 is considered statistically significant.
The baseline, prenoxious, and postnoxious variables were evaluated (Table 2) for both groups. The baseline hemodynamic values before the induction were comparable between the two groups (Table 2). At age-corrected 1.0 MAC, there was no significant difference in the prestimulus hemodynamic variables between both the groups (HR p = 0.06, MAP p = 0.17). Similarly, there was no statistically significant difference in the prestimulus RE, SE, RE–SE between the isoflurane and sevoflurane groups (RE p = 0.46, SE p = 0.07, RE–SE p = 0.06; Table 2).
|
Variables (mean ± SD) |
Group 1 (isoflurane) |
Group 2 (sevoflurane) |
p-Value |
|---|---|---|---|
|
Baseline |
|||
|
HR (bpm) |
88 ± 20 |
80 ± 14 |
0.15 |
|
MAP (mm Hg) |
94 ± 10 |
96 ± 12 |
0.5 |
|
RE |
96 ± 2 |
95 ± 3 |
0.3 |
|
SE |
90 ± 3 |
89 ± 2 |
0.3 |
|
RE–SE |
6 ± 1 |
6 ± 1 |
1 |
|
Prenoxious stimulus |
|||
|
HR (bpm) |
78 ± 12 |
71 ± 10 |
0.06 |
|
MAP (mm Hg) |
85 ± 8 |
81 ± 8 |
0.17 |
|
RE |
38 ± 5 |
37 ± 5 |
0.46 |
|
SE |
38 ± 5 |
35 ± 4 |
0.07 |
|
RE–SE |
0.2 ± 3 |
1.7 ± 2 |
0.06 |
|
Postnoxious stimulus |
|||
|
HR (bpm) |
103 ± 15 |
95 ± 9 |
0.05a |
|
MAP (mm Hg) |
100 ± 8 |
97 ± 9 |
0.39 |
|
RE |
59 ± 5 |
56 ± 5 |
0.06 |
|
SE |
48 ± 5 |
46 ± 5 |
0.12 |
|
RE–SE |
11 ± 3 |
10.45 ± 4 |
0.63 |
Abbreviations: bpm, beats per minute; HR, heart rate; MAP, mean arterial pressure; RE, response entropy; SE, state entropy.
During the poststimulus period, there was a significant increase in HR in the isoflurane group compared with the sevoflurane group (103 ± 15 vs. 95 ± 9, p = 0.05) (Table 2). However, there was no significant difference in the MAP values between both groups (p = 0.39). Though the SE, RE, and RE–SE increased linearly during the poststimulus period in both the groups, there was no statistically significant difference between the two groups (SE p = 0.12, RE p = 0.06, RE–SE p = 0.63) (Table 2). RE–SE was > 10 in both the isoflurane and sevoflurane groups (Table 2). RE–SE was 10.45 ± 4 in the sevoflurane group compared with 11 ± 3 in the isoflurane group (p = 0.63).
Discussion
The main aim of our study was to compare the analgesic and hypnotic potencies of isoflurane and sevoflurane at age-corrected 1.0 MAC in response to a standardized noxious stimulus. We found that isoflurane and sevoflurane had comparable analgesic and hypnotic potencies before and after providing the noxious stimulus.
Electroencephalographic (EEG) activity can assess and measure the hypnotic states and the cortical response to noxious stimulation.6 Time–frequency balanced spectral entropy (M-Entropy) is one of the EEG-based monitors used to monitor the neurophysiological response to anesthesia. The SE index is calculated for frequencies between 0.8 and 32 Hz, reflecting the EEG activity and the time windows for measuring the SE index vary between 15 and 60 seconds. The RE index is calculated for frequencies between 0.8 and 47 Hz and reflects EEG and frontal electromyographic (fEMG) activity. The time windows for measuring the SE index vary between 2 and 15 seconds. The difference between RE and SE indicates EMG activity alone and can be a measure of nociceptive–antinociceptive balance.7 We used entropy indices as surrogate measures for hypnosis and analgesia.
The potential of entropy monitor to reflect nociceptive–antinociceptive balance has been explored by many studies in the past. Guerrero et al, in their observational, prospective study using 3 and 4% sevoflurane anesthesia, found that the difference between RE and SE increased significantly after a noxious stimulus.8 Aho et al also had similar results wherein deepening the plane of anesthesia produced a simultaneous decrease of RE and SE values with a concurrent dominance of low-frequency EEG. However, the application of a noxious stimulus induced an increase in RE and RE–SE values.5 Wheeler et al, using isoflurane anesthesia, observed that noxious stimulus was associated with a concurrent increase in SE and RE and increased autonomic responses in terms of HR and BP. They also noticed that RE increased initially followed by SE, supporting the relationship between fEMG and patient arousal. In the calculation of RE, 2 seconds is used to assess fEMG compared with 15 to 60 seconds for measuring brain electrical activity; this could be the plausible reason for the delay in the rise of SE.9
Our study is the first to compare the analgesic potency of isoflurane and sevoflurane with entropy indices. RE and SE increased after the noxious stimulus, and RE–SE was > 10 in both the isoflurane and sevoflurane groups. These findings are similar to that of Mathews, who successfully integrated RE–SE into an automated algorithm for opioid administration.10 It further concurred with the results of Gruenewald and Ilies, where RE–SE of <10 was associated with a significant reduction in opioid consumption.11
The similarity noted in the entropy indices of sevoflurane and isoflurane could be due to the similar EEG effects of these agents. Schwender et al calculated the spectral edge frequency (SEF), total power, and relative power of the delta, theta, α, and β in the EEG. They found that at equi-MAC levels, isoflurane and sevoflurane had equipotent EEG suppression.12 Rehberg obtained concentration-response curves for these agents and compared the dose for SEF reduction with equi-MAC values. Their findings also demonstrated that the EEG-slowing effect of these agents is not different from the potency measured by MAC.13
We used the entropy indices to compare the hypnotic potencies of isoflurane and sevoflurane at equi-MAC values. Our study results have shown that both SE and RE were comparable in the isoflurane and sevoflurane groups at an age-corrected MAC of 1.0. Eger defined MAC as the minimum alveolar concentration of inhaled anesthetic required to prevent 50% of subjects from responding to a standard painful stimulus (initial skin incision) with “gross purposeful movement.”2 However, one major limitation in using MAC as hypnotic potency is that this “gross purposeful movement” is produced at the spinal (motor neuron) level and is independent of cerebral function. Thus, the MAC awake concept was introduced as the monitor of cerebral cortical state.14 MAC awake expresses the anesthetic concentration at which consciousness might be regained; it was defined as the anesthetic concentration needed to suppress a voluntary response to verbal command in 50% of patients. The ratio of MAC awake to MAC describes the emergence from anesthesia.3
We compared sevoflurane and isoflurane as these both agents had a similar MAC awake to MAC ratio. It is noted that isoflurane, sevoflurane, and desflurane have comparable MAC awake/MAC ratios. The ratio is higher for halothane, which is reflected by studies showing increased bispectral index (BIS) and entropy values for halothane compared with isoflurane and sevoflurane at similar anesthetic concentrations.15 Therefore, further studies are required to elucidate whether agents with comparable MAC awake/MAC ratios have similar potencies with respect to hypnosis, analgesia, and immobilizing properties.
The results of our study should be interpreted keeping in mind the limitations of entropy monitor as surrogate measures of hypnosis and analgesia. In one study, there was an increase of SE, RE, and RE–SE during intubation in patients receiving propofol anesthesia. They analyzed the absolute values and the raw EEG data and found that the increase of RE was soon followed by an increase in SE values, decreasing the RE–SE difference. They presumed that the cause of the rise in SE was not because of EEG activation but could be attributed to the intense EMG activity changing the EEG spectrum at 20 Hz.16 It must be noted that all activity below 32 Hz is regarded as EEG in entropy analysis. It is possible that SE can capture the EMG activity as there is an overlap of the frequencies captured by SE and RE. However, there was an increase in SE and RE in our study with a concomitant increase in RE–SE above 10 during the noxious stimulus.
Few studies have shown a lack of RE to painful stimulus in fully paralyzed patients. Kawaguchi et al showed that muscle relaxants suppressed the change in entropy to intubation in a dose-dependent manner.17 Xing et al found that muscle relaxants significantly reduced spectral entropy changes in response to a noxious stimulus.18 These studies suggest that the values of RE and RE–SE are purely EMG related, and consequently, these indices are unreliable in a complete or incomplete neuromuscular blockade. We ensured a TOF of 4 prior to recording prenoxious and postnoxious values to remove the confounding factor of effects of neuromuscular blockade on the study.
We had compared the effects of isoflurane and sevoflurane at 1.0 MAC and found that they produce similar entropy indices. Similarly, Kim et al reported similar BIS values for these agents at 1 MAC.19 However, another study demonstrated that sevoflurane produced lesser BIS/more hypnosis than isoflurane both at wash-in and wash-out phases of anesthesia.20 Kurehara et al evaluated the BIS values in patients anesthetized with sevoflurane and isoflurane. They found that at 1.2 MAC, both anesthetics produced the same BIS values. However, increasing the anesthetic concentration to 2.0 MAC decreased the BIS values in patients anesthetized with isoflurane; however, it had no effect in those anesthetized with sevoflurane.21 Moreover, it is to be noted that a study by Ryu et al, which compared desflurane and sevoflurane (agents with similar MAC awake/MAC ratio), found that they produced different levels of hypnosis as measured by BIS.22 Further studies comparing these agents' hypnotic and analgesic effects at increasing MAC levels would throw light on this.
Randomized double-blind trials are considered a superior study design since randomization, and double blinding eliminates confounding variables.23 In our study, though patients were blinded to the agent they were administered, the anesthetists who provided anesthesia and recorded the values were not blinded. This could have led to bias, though strict study protocol adherence and withdrawal criterion was followed in the study. Also, the primary outcome of interest was the measure of analgesia, and the sample size was calculated based on the same and not for the measure of hypnosis. However, the SE (a measure of hypnosis) of isoflurane and sevoflurane in the study was 38 ± 5 and 35 ± 4 and the sample size for a two-sided t-test with a power of 80%, and a significance level of 5% is 43. Hence, we believe the study results are valid not just for the measure of analgesia but also for hypnosis.
Conclusion
In conclusion, our study shows that during a steady state of age-corrected 1.0 MAC single-agent anesthesia, sevoflurane and isoflurane have comparable analgesic and hypnotic potencies as measured by entropy indices when a standardized nociceptive stimulus is provided.
Conflict of Interest
None declared.
Funding None.
References
- Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127(5):1246-1258.
- [Google Scholar]
- Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake. Anesth Analg. 2001;93(4):947-953.
- [Google Scholar]
- Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68(5):512-522.
- [Google Scholar]
- Effect of an intubation dose of rocuronium on spectral entropy and bispectral index responses to laryngoscopy during propofol anaesthesia. Br J Anaesth. 2006;97(6):842-847.
- [Google Scholar]
- Explaining entropy responses after a noxious stimulus, with or without neuromuscular blocking agents, by means of the raw electroencephalographic and electromyographic characteristics. Br J Anaesth. 2011;106(1):69-76.
- [Google Scholar]
- Assessment of nociceptive responsiveness levels during sedation-analgesia by entropy analysis of EEG. Entropy (Basel). 2016;18(3):103.
- [Google Scholar]
- Monitoring the nociception-anti-nociception balance. Best Pract Res Clin Anaesthesiol. 2013;27(2):235-247.
- [Google Scholar]
- Response entropy changes after noxius stimulus. J Clin Monit Comput. 2012;26(3):171-175.
- [Google Scholar]
- Response entropy increases during painful stimulation. J Neurosurg Anesthesiol. 2005;17(2):86-90.
- [Google Scholar]
- Response entropy-state entropy difference and nociception: a matter of context. Br J Anaesth. 2009;103(1):135-136. , author reply 136–137
- [Google Scholar]
- M-Entropy guidance vs standard practice during propofol-remifentanil anaesthesia: a randomised controlled trial. Anaesthesia. 2007;62(12):1224-1229.
- [Google Scholar]
- Power spectral analysis of the electroencephalogram during increasing end-expiratory concentrations of isoflurane, desflurane and sevoflurane. Anaesthesia. 1998;53(4):335-342.
- [Google Scholar]
- Comparative pharmacodynamic modeling of the electroencephalography-slowing effect of isoflurane, sevoflurane, and desflurane. Anesthesiology. 1999;91(2):397-405.
- [Google Scholar]
- EEG entropy values during isoflurane, sevoflurane and halothane anesthesia with and without nitrous oxide. J Neurosurg Anesthesiol. 2009;21(2):108-111.
- [Google Scholar]
- Facial muscle activity, response entropy, and state entropy indices during noxious stimuli in propofol-nitrous oxide or propofol-nitrous oxide-remifentanil anaesthesia without neuromuscular block. Br J Anaesth. 2009;102(2):227-233.
- [Google Scholar]
- Rocuronium dose-dependently suppresses the spectral entropy response to tracheal intubation during propofol anaesthesia. Br J Anaesth. 2009;102(5):667-672.
- [Google Scholar]
- Effects of neuromuscular blockages on entropy monitoring during sevoflurane anesthesia. Med Sci Monit. 2019;25:8610-8617.
- [Google Scholar]
- Relationship of bispectral index to minimum alveolar concentration during isoflurane, sevoflurane or desflurane anaesthesia. J Int Med Res. 2014;42(1):130-137.
- [Google Scholar]
- Comparison of equi-minimum alveolar concentration of sevoflurane and isoflurane on bispectral index values during both wash in and wash out phases: a prospective randomised study. Indian J Anaesth. 2015;59(2):79-84.
- [Google Scholar]
- [Relationship between minimum alveolar concentration and electroencephalographic bispectral index as well as spectral edge frequency 95 during isoflurane/epidural or sevoflurane/epidural anesthesia] Masui. 2001;50(5):512-515.
- [Google Scholar]
- Does equi-minimum alveolar concentration value ensure equivalent analgesic or hypnotic potency?: A comparison between desflurane and sevoflurane. Anesthesiology. 2018;128(6):1092-1098.
- [Google Scholar]
- Randomized double blind placebo control studies, the “Gold Standard” in intervention based studies. Indian J Sex Transm Dis AIDS. 2012;33(2):131-134.
- [Google Scholar]






