Translate this page into:
Detailed Clinical and Electrophysiological Illustration of a Patient with Sturge–Weber Syndrome Presenting with Prolonged Transient Neurological Symptoms
Address for correspondence: Dr. Halil Onder, Neurology Clinic, Yozgat City Hospital, Yozgat, Turkey. E-mail: halilnder@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although transient neurological deficits in Sturge–Weber syndrome (SWS) have been acknowledged for many years, the underlying pathogenesis still constitutes a major topic of discussion. Here, we present the case of a 10-year-old boy with SWS presenting with a progressive clinically mimicking right middle cerebral artery syndrome. Cranial magnetic resonance imaging showed unremarkable findings except extensive right hemisphere leptomeningeal angiomas. Antiepileptic drug (AED) treatments provided recovery of the patient, and concurrently recorded electroencephalography (EEG) revealed delta slowing of the lesional hemisphere in the acute period. However, slowing of the background activity extended to the contralateral hemisphere in subacute period, while ipsilateral background activity started to recover. In the 3rd week of follow-up on intensive AED treatments, the patient totally recovered as well as nearly the normalization of EEG was achieved. In this report, we focus on the dramatic revolution of EEG activity in both hemispheres during the recovery period. Based on this rare illustration and limited literature data, the author suggests some hypotheses regarding the pathophysiology of this mysterious manifestation (transient neurological deficit) in SWS. Future studies of large case series, including detailed paraclinical parameters and remarkably electrophysiological data, are warranted to clarify these arguments.
Keywords
Electroencephalography
ictogenesis
pathogenesis
Sturge–Weber syndrome
transient neurological symptoms
INTRODUCTION
Sturge–Weber syndrome (SWS) is a congenital neurocutaneous disorder characterized by facial cutaneous nevus, leptomeningeal angioma, and glaucoma.[1] Epilepsy is the most common symptom in SWS.[2] Besides, transient neurological deficits in SWS have been acknowledged for many years.[3456] Usually, ictal and postictal depression of activity have been suggested as the responsible mechanisms of transient neurological symptoms in SWS.[2] However, mechanisms other than ictogenesis such as ischemia[4] and migraine pathophysiology[5] have recently been associated with these symptoms in SWS. In this report, we illustrate an interesting patient of SWS presenting with clinically mimicking right middle cerebral artery syndrome. Through detailed clinical and electroencephalography (EEG) follow-up of this patient, we discuss the possible pathomechanisms underlying these mysterious symptoms of transient neurological deficits in SWS.
CASE REPORT
A 10-year-old boy presented with complaints of left-sided weakness and lisping developing over the last 1 day in a progressive manner. Upon interrogation of medical history, it was learned that he was noticed to have a right facial port-wine lesion at birth and he had developed a focal seizure first at the age of 8 months. Following brain magnetic resonance imaging (MRI) recording, which had revealed extensive leptomeningeal angioma with associated cerebral atrophy on the right hemisphere and subcortical calcifications in the frontoparietal lobe, the diagnosis of SWS was made and a close follow-up had been conducted in another neurology clinic for seizures and also in an ophthalmology clinic for right congenital glaucoma. He had no seizure since 3 years and he was on treatments of 1000 mg levetiracetam and 200 mg phenytoin on admission to our center. Remarkably, it was learned that similar left-sided paralysis attacks had also previously occurred at 2 years and 3 years.
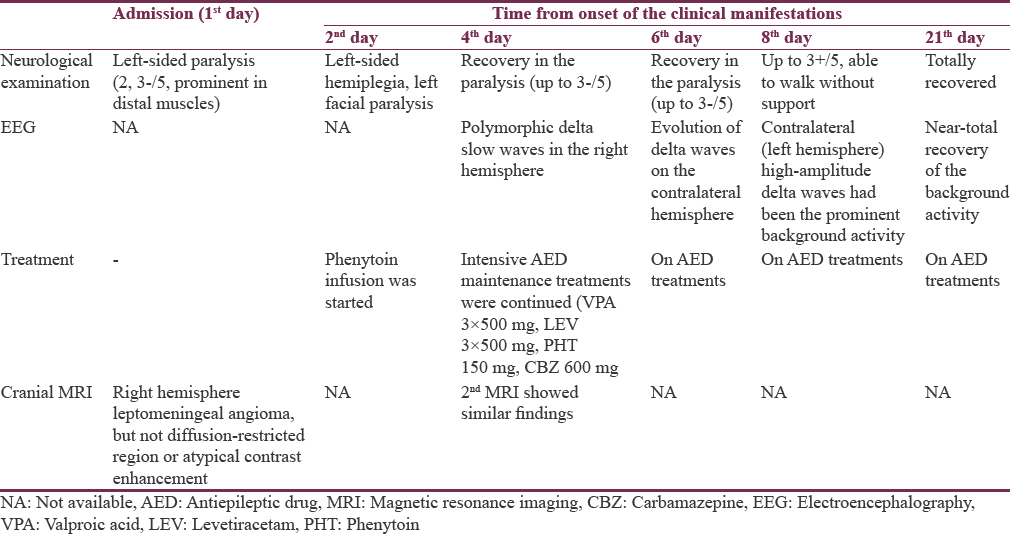
On neurological examination at admission, he was conscious and orientated; however, left central facial paralysis and dysarthria were present. Motor examinations showed weakness (2/5 on Medical Research Council scale) of left upper and lower limbs, predominant in distal muscles. Other examinations were within normal ranges, and neck stiffness was absent (Kernig's and Brudzinski's signs were negative). Vital signs revealed a mild fever (37.2°C). Cranial MRI showed right hemisphere leptomeningeal angioma, but not diffusion-restricted region or atypical contrast enhancement [Figure 1]. Other laboratory investigations were unremarkable, except the findings of urinary tract infection (pyuria). On the 2nd day of admission, motor weakness worsened to the degree of total hemiplegia and left facial paralysis progressed. EEG could not be performed due to technical problems (the patient was admitted at the weekend and EEG technicians were not on duty). However, taken together the clinical presentations and negative MRI findings, the diagnosis of status epilepticus with negative phenomenon was considered in the forefront. Supporting this diagnosis, phenytoin loading therapy (18 mg/kg) provided a prompt moderate recovery for left paralysis in the acute period (up to 3/5 of upper distal extremity muscles). In the following course, antiepileptic drug (AED) maintenance therapies were increased up to the dosages of valproic acid 3 × 500 mg, levetiracetam 3 × 500 mg, phenytoin 150 mg/day, and carbamazepine 600 mg/day (the patient weighed 40 kg). EEG (NIHON KOHDEN Neurofax EEG-9000 UNIT) was done on the 4th day of clinical manifestations which showed polymorphic delta slow waves in the right hemisphere, but no ictal recording [Figure 2]. However, AED regimens were continued and, under these treatments, the patient improved gradually and, on the 6th day of the event, he was able to walk without support. On the other hand, concurrently performed EEG showed distribution of delta waves to the contralateral hemisphere (left hemisphere) and a mild recovery of the background activity in the lesional hemisphere [Figure 3]. On the 8th day, EEG showed that the background activity of high-amplitude delta waves was prominent in the left hemisphere [Figure 4]. At polyclinic follow-up on the 21th day, he was evaluated to be totally recovered. EEG revealed near-total recovery of the background activity [Figure 5].
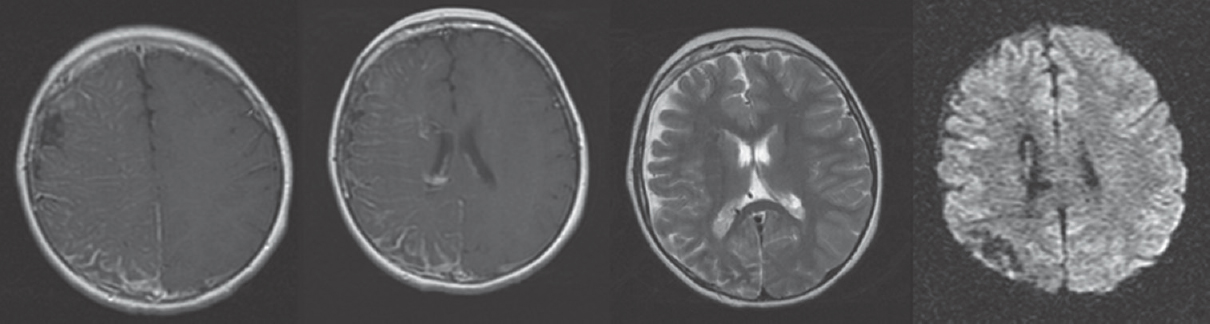
- Cranial magnetic resonance imaging showing leptomeningeal contrast enhancement compatible with leptomeningeal angiomas. Diffusion weighted imaging showing normal appearance
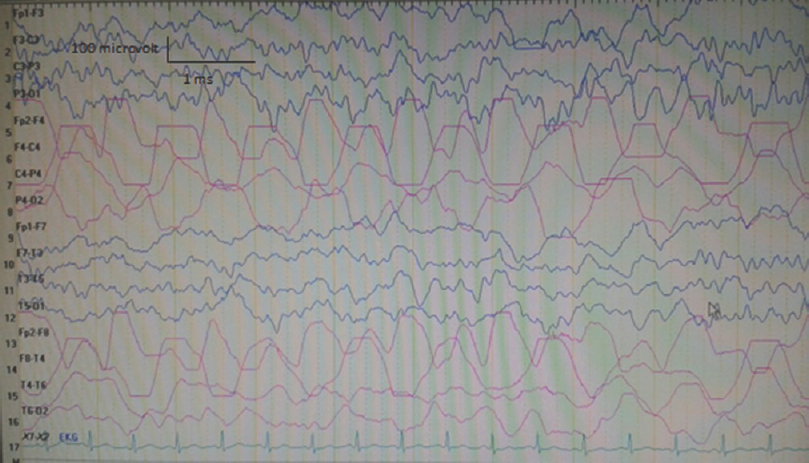
- Electroencephalography (double banana 1) recorded on the 3rd day of admission showing polymorphic delta slow waves in the right hemisphere (concurrently neurological examination revealed 3-/5 motor paralysis in the left upper and lower limbs)
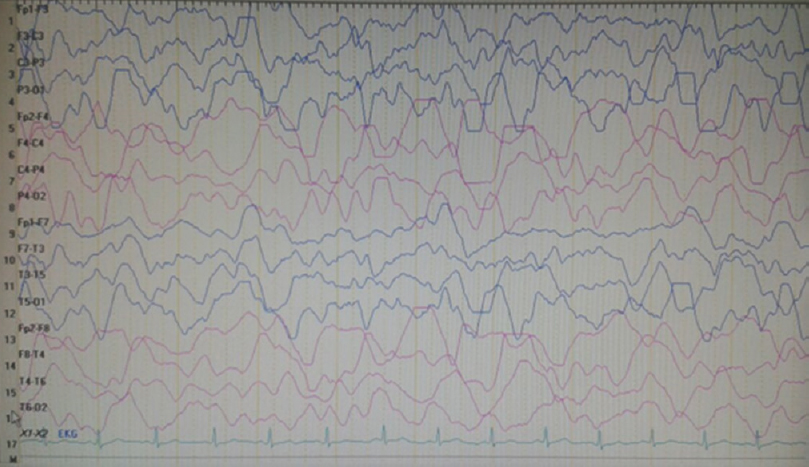
- Electroencephalography recorded on the 5th day of admission showing evolution of delta waves on the contralateral hemisphere (left hemisphere) and a mild increment in the background delta activity of the right hemisphere
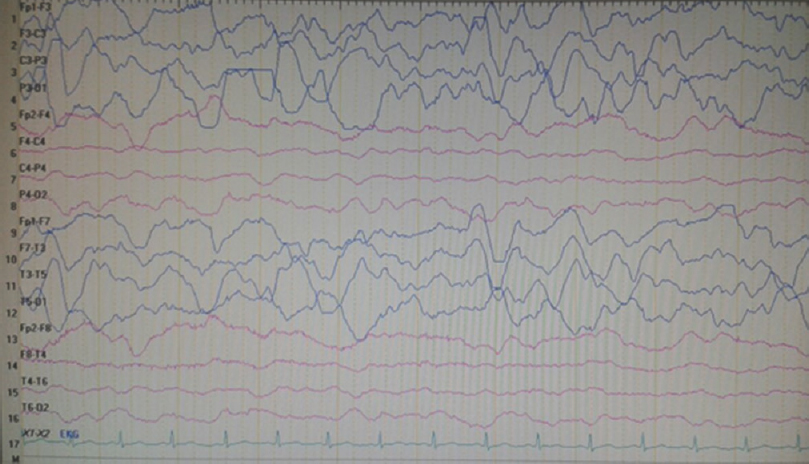
- Electroencephalography, on the 7th day of admission, showing right hemisphere voltage attenuation and resolution of delta waves, whereas contralateral (left hemisphere) high-amplitude delta waves had been the prominent background activity
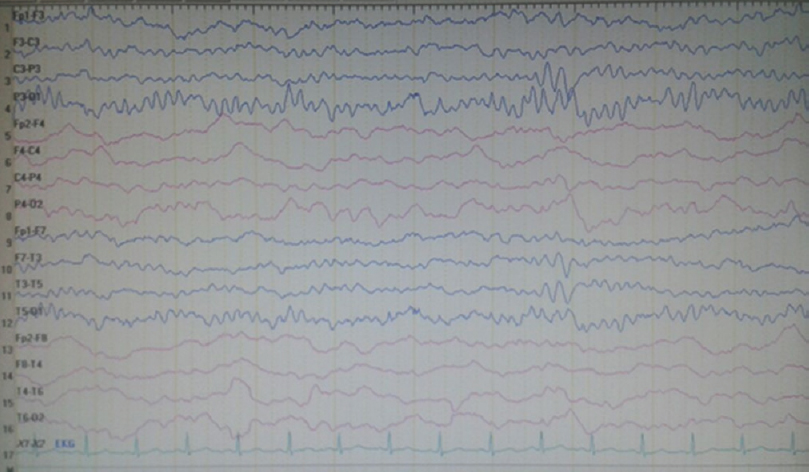
- Electroencephalography recorded on the 20th day of admission showing near-total recovery of the background activity (concurrently motor strength examinations showed normal muscle strength and other neurological evaluations were within normal ranges)
DISCUSSION
Although several hypotheses have been postulated to explain the mechanisms of ictogenesis and transient neurological symptoms in SWS, the underlying mechanisms still remain to be clarified. An important hypothesis was based on an interesting observation of paradoxical EEG pattern in SWS which was called “steal phenomenon.”[7] In this report by Limotai et al., the authors referred to a previous study, emphasizing the knowledge of impaired brain perfusion in SWS and excessive metabolic demand in prolonged seizure activity, which can result in exacerbation of ischemic brain injury. In conclusion, they suggested that both of these mechanisms might be playing a role in the manifestations of neurological deteriorations in children with SWS.[8] In the report by Limotai et al., two cases with SWS, developing hypomotor seizures, were presented, in whom video EEG monitorization data showed contralateral hemisphere slow waves preceding the ictal EEG change over the lesional hemisphere.[7] However, we presume that there are some points to be focused on when interpreting the presentation of our patient [Table 1]. First, EEG recordings in the acute period had showed slowing of the background activity in the lesional hemisphere, whereas slowing of the background activity was found to be present in the contralateral hemisphere in cases of Limotai et al. However, we found that slow background activity had extended to the contralateral hemisphere, whereas background activity of the lesional hemisphere tended to recover in the subacute chronic period. Second, our patient had presented with negative phenomenon status epilepticus, which was distinct from the cases of Limotai et al. and totally more extreme.
Prolonged transient neurological deficits in SWS have been reported several times in the literature.[569] In the interesting report by Freilinger et al., a patient presenting with prolonged neurological symptoms in the setting of SWS was illustrated. Their patient was admitted with clinically mimicking dense left hemisphere syndrome, proceeding for 10 days and perfusion imaging methods showed impaired left hemispheric perfusion (lesional hemisphere), whereas hypermetabolism of the left hemisphere was detected on the 7th day. In this report, EEG had showed hemispheric slowing in the lesional side in acute phase, but detailed data about follow-up EEGs were not mentioned. We believe that the underlying pathomechanisms of hypometabolism following hypermetabolism, shown in this patient via perfusion imaging methods, may also give crucial views regarding the mechanisms of these clinical manifestations similar to the illustration of our patient. Although perfusion investigations were not performed in our case, detailed EEG findings of our case may probably add crucial perspectives to the knowledge of transient neurological deficits in SWS patients.
Another topic of discussion has been focused on the possible association of these symptoms and migraine pathophysiology.[5] Freilinger et al. explained clinical manifestations of their patient in the setting of migraine pathophysiology. They based their view on specific features of headache manifesting in their patient and unremarkable EEG findings. Nonetheless, in this report, the patient was reported to suffer from a clonic seizure in the following period and clinical recovery had been achieved following phenytoin administration which can rather be attributed to an underlying ictogenesis. The authors did not postulate any discussions focusing on this point, which may be a major limitation of this report. In our patient, interestingly, no EEG recordings showed an ictal pattern, similarly. However, loading dose of phenytoin infusion (15 mg/kg) had promptly resulted in a significant improvement of the neurological status of the patient. In addition, the patient had no headache, and a history of a previous migraine attack had not been taken. Of note, EEG recordings were unavailable in the acute period of clinical event, considering the possibility that ictogenesis could not be detected due to this technical limitation in the acute period. In accordance with this thought, it has also been previously reported that scalp EEG might be insufficient to provide definitive information to determine the severity of epilepsy and origin and propagation of epileptic discharges in SWS.[410] It has been suggested that thick leptomeningeal angioma covering cerebral cortex enables the chance of detecting the low-amplitude ictal and/or interictal epileptic discharges.[11] Hence, we presume that the basic mechanism playing a role in this mysterious manifestation might be associated with an underlying ictogenesis that probably could not be detected by routine EEG investigations. Besides, recently, studies using intracranial subdural electrodes and depth electrodes demonstrated seizures initiated from the cortex under the leptomeningeal angioma.[1112] Furthermore, based on the location of seizure initiation region (under angioma), they pointed out ischemia as a principal mechanism underlying ictogenesis and epileptogenesis in SWS.[11]
At this point, a very interesting report by Bello-Espinosa et al. gives substantial perspectives to the knowledge of this issue.[13] In this report, the case of a child with SWS and subclinical status epilepticus was demonstrated, in whom EEG had showed asymmetric slowing, but no ictal activity. Meanwhile, using low-frequency filter (LFF), 0.01 Hz, and high-frequency filter (HFF), 0.1 Hz, continuous prolonged rhythmic ictal infraslow activity was demonstrated in the lesional hemisphere. Midazolam infusion made possible the cessation of the infraslow activity, considering an underlying ictogenesis. Based on the previous reports, emphasizing healthy and sick astrocytes as the responsible factors in the mechanism of infraslow activity,[14] the authors attributed these activities to a probable disruption of glial network or nonneuronal component. Unfortunately, our EEG machine cannot designate the setting of LFF: 0.01 Hz and HFF: 0.1 Hz to determine infraslow activity. Hence, the possible role of infraslow activity in the clinical course of our patient cannot be excluded, which may also constitute a crucial argument. Furthermore, we believe that infraslow activity may reflect an extremely important issue as a mechanism underlying ictogenesis in SWS as well as other etiologies of suspected status epilepticus which should be focused on in the future studies.
Taken together the literature knowledge and the clinical course of our patient, we think that delta wave slowing in the lesional hemisphere determined in the acute period might be reflecting an underlying ischemia and associated ictogenesis. However, mechanisms underlying contralateral slowing of the background activity developing mainly in the subacute period constitute quite interesting aspects for further deliberations. A possible hypothesis may be the “steal phenomenon,” as suggested similarly by Limotai et al.[7] Based on the clinical and laboratory findings of our patient, we hypothesize that AED administration might have influenced via ameliorating the ictogenesis that probably could not be determined by routine EEGs. Meanwhile, we think that slowing of the background activity in the lesional hemisphere might be reflecting pathological ischemia associated with ictogenesis as previously suggested.[8] Besides, contralateral hemisphere delta slowing in the following period might be reflecting the compensation of ischemic changes at the side of the angioma by contralateral hemisphere cerebral blood flow, similar to the hypothesis of Limotai et al. This consideration is also consistent with the results of perfusion scanning of a previous study[5] showing pathological hypometabolism following hypermetabolism in the lesional hemisphere.
CONCLUSION
We hypothesize that both ictogenesis and associated perfusion insufficiency may be involving in the pathological mechanisms of prolonged transient neurological deficits in SWS. However, future large studies of case series using multiple paraclinical methods (imaging, EEG) have to be conducted to clarify these arguments.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Sturge-Weber syndrome and paroxysmal hemiparesis: Epilepsy or ischaemia? Dev Med Child Neurol. 2004;46:783-6.
- [Google Scholar]
- Recurrent thrombotic deterioration in the Sturge-Weber syndrome. Childs Brain. 1981;8:427-33.
- [Google Scholar]
- Diazepam-enhanced beta activity in Sturge Weber syndrome: Its diagnostic significance in comparison with MRI. Clin Neurophysiol. 2002;113:1025-9.
- [Google Scholar]
- A case of Sturge-Weber syndrome with symptomatic hemiplegic migraine: Clinical and multimodality imaging data during a prolonged attack. J Neurol Sci. 2009;287:271-4.
- [Google Scholar]
- High prevalence of bihemispheric structural and functional defects in Sturge-Weber syndrome. J Child Neurol. 1998;13:595-605.
- [Google Scholar]
- Steal phenomenon in Sturge-Weber syndrome imitating an ictal electroencephalography change in the contralateral hemisphere: Report of 2 cases. J Neurosurg Pediatr. 2015;16:212-6.
- [Google Scholar]
- Sturge-Weber syndrome: Cerebral haemodynamics during seizure activity. Dev Med Child Neurol. 1999;41:480-5.
- [Google Scholar]
- Central nervous system structure and function in Sturge-Weber syndrome: Evidence of neurologic and radiologic progression. J Child Neurol. 1998;13:606-18.
- [Google Scholar]
- The epilepsy of Sturge-Weber syndrome: Clinical features and treatment in 23 patients. Acta Neurol Scand Suppl. 1992;140:18-22.
- [Google Scholar]
- Analysis of epileptic discharges from implanted subdural electrodes in patients with Sturge-Weber syndrome. PLoS One. 2016;11:e0152992.
- [Google Scholar]
- Intraoperative electrocorticography and successful focus resection in a case of Sturge-Weber syndrome. Seizure. 1998;7:505-8.
- [Google Scholar]
- Infraslow status epilepticus: A new form of subclinical status epilepticus recorded in a child with Sturge-Weber syndrome. Epilepsy Behav. 2015;49:193-7.
- [Google Scholar]
- Infraslow (<0.1 Hz) oscillations in thalamic relay nuclei basic mechanisms and significance to health and disease states. Prog Brain Res. 2011;193:145-62.
- [Google Scholar]






