Translate this page into:
An Intractable Case of Anti-N-methyl-D-aspartate Receptor Encephalitis with Prolonged Hyperammonemia
Rajesh Verma, DM, DNB Department of Neurology, King George's Medical University Lucknow, Uttar Pradesh 226003 India drrajeshverma32@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Introduction
Acute encephalitis is a life-threatening neurological illness that presents as a rapidly progressive encephalopathy due to inflammation of the brain.1 In the recent past, in addition to infectious causes, various autoantibody-mediated encephalitis has been identified. There are two types of immune-mediated encephalitis, namely, paraneoplastic encephalopathy syndromes related to cancers and antibody perturbing neuronal surface proteins or antigens of synapses.2 Autoimmune encephalitis has distinct categories such as anti-N-methyl-D-aspartate receptor (anti-NMDAR), leucine-rich glioma-inactivated protein 1, and contactin-associated protein-2.3 Autoimmune encephalitis is a specific syndrome with characteristic clinical features. Anti-NMDAR encephalitis is a subtype of autoimmune encephalitis occurring due to autoantibodies targeting NMDARs either due to ovarian teratoma or preceding viral infections acting as triggers.4
Nonhepatic hyperammonemia occurs due to disorders not involving hepatic pathways directly. Inadequate dietary intake in intensive care unit (ICU) patients can precipitate hyperammonemia encephalopathy, which can exacerbate the ill effects of preexisting illness.5 In this case illustration, we presented a patient of anti-NMDAR encephalitis treated with sequential immunomodulatory drugs. The case had prolonged hyperammonemia contributing to a longer duration of illness, and corrective measures led to complete recovery. We emphasized that ammonia levels should be assessed in treating patients with autoimmune encephalitis.
Case History
A 10-year-old boy presented with nonspecific pain 4 months before the onset of illness. After 15 days, he developed multiple convulsions of complex partial type with automatisms, occurring two to three episodes every second to third day. The seizures gradually increased in frequency. The local clinician started antiepileptics in the form of valproate and clobazam at appropriate dosages. There was partial control of the seizures. It was followed by headache, recurrent vomiting, and insomnia with personality and behavior changes.
He was restless, agitated, unwarranted, running here and there with reversal of sleep-wake pattern. He used to abuse his family members and even treating physicians. He had aggressive behavior and social-disinhibited features with urination at inappropriate places and occasionally getting naked in front of people. On examination, he was agitated with paranoid behavior. His detailed mental status examination could not be done because of his insufficient attention. The cranial nerves, motor, reflexes, and cerebellar examination were normal. Clinically, he had subacute encephalitic features with marked psychiatric manifestations.
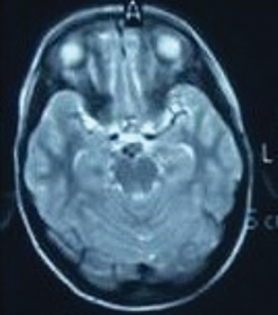
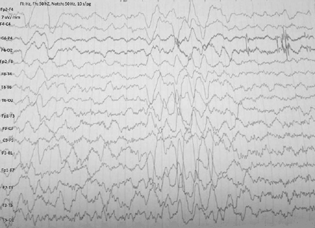
The investigative parameters including hematological, renal, and liver function tests were normal. The thyroid functions, including thyroid peroxidase and thyroglobulin antibodies were within normal limits. The autoimmune markers including antinuclear antibody and rheumatoid factor did not reveal any abnormality. He was negative for viruses causing encephalitis, namely, Japanese encephalitis, herpes simplex viruses, cytomegalovirus, and dengue virus. Magnetic resonance study of the brain revealed subtle hyperintensities in medial temporal lobes (Fig. 1). His electroencephalography revealed an intermittent burst of epileptiform discharges along with delta-brush pattern (Fig. 2). Serum ammonia at the time of admission was elevated 140 µ/dL (normal range: 15–45 µ/dL). After 15 days, it was still elevated (61 µ/dL). The cerebrospinal fluid anti-NMDAR antibody titer was strongly positive. The cerebrospinal fluid, cytological and biochemical study showed two cells, all lymphocytes, protein 65 mg/dL and sugar 38 mg/dL.
-
Fig. 1 Magnetic resonance imaging of cranium revealed subtle hyperintensities in medial temporal lobes.
Fig. 1 Magnetic resonance imaging of cranium revealed subtle hyperintensities in medial temporal lobes.
-
Fig. 2 Electroencephalography demonstrated an intermittent burst of generalized epileptiform discharges along with delta brush pattern.
Fig. 2 Electroencephalography demonstrated an intermittent burst of generalized epileptiform discharges along with delta brush pattern.
The patient was diagnosed as anti-NMDAR encephalitis, and he was administered intravenous infusion of methylprednisolone 1 g/d for 5 days. But the patient did not improve with persistent behavioral abnormality for which immunoglobulin therapy (0.4 g/kg body weight per day) was given for 5 days. The patient still had features of motor agitation, intelligible speech, and change in personality and behavior. Psychiatrist consultations were done several times for his psychotic behavior, and they advised antipsychotic drugs. Amazingly, we checked serum ammonia levels for multiple times, and it was elevated till the last report and treatment was given for the same. We administered him rituximab therapy weekly for a month. His psychiatric manifestations disappeared. He became completely asymptomatic at 6 months and resumed all his activities of daily living.
Discussion
Encephalitis is inflammation of the brain parenchyma due to varied etiology. The cardinal features of acute encephalitis are fever, headache, vomiting, seizures, and impaired sensorium. The common etiological factors are infections, inflammatory diseases, demyelinating diseases, and vasculitis.1
In the recent past, various autoantibodies have been searched causing autoimmune encephalitis. Autoimmune encephalitis has been classified into two categories. The intracellular neural antigens can be targeted by various onconeural antigenic antibodies causing paraneoplastic syndromes. Paraneoplastic syndromes are usually associated with cancers.6 The other subset of autoimmune encephalitis occurs due to antibodies targeting cellular membrane or synapses subserving receptors for various neurotransmitters. The commonly described autoantibody-induced encephalitis are NMDAR, LG11 (leucine-rich glioma-inactivated protein 1), CASPR (Contactin-associated protein-2), and DIPX (dipeptidyl-peptidase–like protein 6)-mediated encephalitic syndromes. These various types of autoimmune encephalitis have specific clinical features.7 Anti-NMDAR encephalitis develops due to antibody targeting NMDAR-mediated neurotransmission. It mainly occurs due to ovarian teratoma, epitopes mimicking NMDAR surface neural antigens, or preceding viral infections, particularly herpes simplex viruses.8
Our patient presented with altered behavior, seizures, agitation, and motor restlessness. The NMDAR encephalitis presents with psychiatric features, aggression, agitation, convulsions, echolalia, and impaired behavior.9 The treatment consists of first-line therapy, including intravenous methylprednisolone, immunoglobulin therapy, and second-line drugs such as B cell depletors (rituximab).10 We treated our patient initially with intravenous methylprednisolone pulse therapy for 5 days along with antiepileptic medications. However, the patient did not respond to treatment for which immunoglobulin therapy was given for 5 days. The patient still had features of motor agitation, intelligible speech, and change in personality and behavior. Hyperammonemia was treated accordingly. We waited further for a fortnight for due clinical response. It prompted us to administer rituximab therapy. Finally, he recovered and regained normal behavior and was free from motor agitation.
The causes of hyperammonemia are hepatic illness (acute or chronic) or nonhepatic conditions. The elevated ammonia level can lead to encephalopathy characterized by impaired mental status, coma, brain edema, and even death. Nonhepatic hyperammonemia can be categorized into mild (36–39 mol/L) or severe (more than 100 mol/L serum ammonia levels). The etiopathogenesis of nonhepatic hyperammonemia could be inappropriate dietary intake in ICU patients causing muscle injury, releasing amino acids into a cycle of gluconeogenesis and substrates for ammonia production.11 In our patient, ammonia levels were remarkably high throughout hospitalization. Whether this elevated ammonia in ICU settings is a cause of increased mortality or epiphenomenon is yet to be determined. However, in patients of autoimmune encephalitis, the management of nonhepatic hyperammonemia should be done to have better clinical outcome.
Acute as well as chronic hyperammonemia can affect neurotransmission. The critical hyperammonemia results in elevated extracellular levels of glutamate in brain with associated activation of NMDAR. The activation of NMDARs results in adenosine triphosphate depletion and ammonia-mediated toxicity. This upregulation of NMDARs results in seizures, cognitive dysfunction, and psychiatric morbidities.12 We postulated that chronic hyperammonemia in our case probably exacerbated the clinical complications and affected the recovery despite judicious use of immunomodulators.
Conclusion
Anti-NMDAR encephalitis is one of the common types of autoimmune encephalitis. Immunomodulators are the mainstay of treatment. In a subset of patients, anti-NMDAR encephalitis is intractable to treat and requires multiple immune therapies. The point of concern is that repeated ammonia levels should be performed and hyperammonemia should to be treated adequately.
Conflict of Interest
None declared.
References
- Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-1128.
- [Google Scholar]
- Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92(7):757-768.
- [Google Scholar]
- Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866-5875.
- [Google Scholar]
- Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochem Int. 2009;55:106-112. (1-3):
- [Google Scholar]
- Diagnosis and management of paraneoplastic neurologic disorders. Curr Treat Options Oncol. 2013;14(4):528-538.
- [Google Scholar]
- Neuronal surface antibody-mediated autoimmune encephalitis. Semin Neurol. 2014;34(4):458-466.
- [Google Scholar]
- Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
- [Google Scholar]
- Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
- [Google Scholar]
- Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull. 2015;39(1):19-23.
- [Google Scholar]
- NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis. 2007;22:321-335. (3-4):
- [Google Scholar]





