Translate this page into:
Serum S100B and NSE Levels Correlate With Infarct Size and Bladder-Bowel Involvement Among Acute Ischemic Stroke Patients
Rajinder K. Dhamija, MD, DNB, FRACP Department of Neurology, Lady Hardinge Medical College, Room No. 1099, Neurology Office New Delhi 110001 India dhamijark@gov.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives Stroke is a major global health concern. Due to limited availability of neuroimaging particularly in rural and regional areas in India as well as its limitation, the interest in use of biochemical markers for stroke diagnosis, severity, and prognosis is increasing. Only a handful of studies on stroke biomarkers have been conducted in India. Hence, this study was conducted to investigate the correlation of serum neuron-specific enolase (NSE) and S100 calcium-binding protein B (S100B) levels with stroke severity according to infarct size in acute ischemic stroke patients.
Material and Methods Sixty stroke patients were recruited for the study and were evaluated. Noncontrast computed tomography (CT) scan of the brain was performed for all patients within 48 hours of onset of symptoms. Infarct volume was measured by evaluating dimensions in three planes on CT head. Serum NSE and S100B levels were measured by commercially available immunoassay kits. Continuous data was represented as mean ± standard deviation. Categorical data was expressed in terms of percentages and proportions. Pearson's correlation coefficient was applied to assess correlation between NSE and S100B and infarct size. Infarct size was classified arbitrarily into three groups according to infarct volume (low, moderate, and large) and analysis of variance was applied for comparing mean S100B and NSE levels in the three groups. To assess the independent predictors of infarct size among stroke cases, multivariate logistic regression analysis was used. Association between serum S100B or NSE levels and clinical features was done by the Mann–Whitney U test.
Results Correlation between serum S100B protein levels and NSE with larger infarct volume was highly significant (r(S100B) = 0.611, p (S100B) < 0.0001; r(NSE) = 0.258, p(NSE) = 0.047). Using multivariate regression analysis, bladder and bowel involvement, prior stroke history, and dyslipidemia among stroke patients correlated with a larger infarct size. Mann–Whitney U test showed both NSE and S100B levels were significantly associated with bladder bowel involvement among stroke cases.
Conclusion There was a positive correlation between serum S100B and NSE levels with infarct size. In addition, bladder-bowel involvement among stroke patients was associated with increased S100B levels. Therefore, levels of protein S100B and NSE may serve as indicator of infarct size and may be predictors of severe clinical presentations of acute ischemic stroke.
Keywords
neuron-specific enolase
stroke biomarkers
S100B
ischemic stroke
infarct size
Introduction
Stroke continues to remain a major global health concern in low- and middle-income countries (LMICs).1 According to a recent systematic analysis of global stroke burden, stroke is the second leading cause of mortality and a leading cause of disability worldwide. The burden portended by stroke has increased in the last 25 years, especially in LMICs, which bear more than 85% of the global stroke burden.2
Due to the huge societal and economic impact, prompt diagnosis and evaluation of stroke severity is the need of the hour. Stroke severity scales like the National Institute of Health Stroke Scale, the Scandinavian Stroke Scale, the Canadian Stroke Scale, Barthel Index, modified Rankin Scale, etc. predominantly employ clinical assessment. Thus, any direct evidence of severity can only be obtained by neuroimaging that enables assessment of infarct size as well as the neuroanatomical region involved. However, neuroimaging too has several limitations. Computed tomography (CT) has a poor sensitivity for diagnosis of acute ischemic stroke (AIS) albeit a high specificity and CT scan may be normal or show subtle changes particularly if conducted early in the time course of AIS.3 Diffusion-weighted imaging (DWI) has an estimated sensitivity of 88 to 100% and specificity of 95 to 100%, but magnetic resonance imaging is limited by its availability and cost particularly in LMICs.4 Additionally, DWI may miss small lesions located at the level of the brainstem that present with ataxic hemiparesis or internuclear ophthalmoplegia.5
In lieu of these limitations, there has been great interest in developing stroke biomarkers which can assist in rapid diagnosis and give an assessment of stroke severity as well.6 Out of the plethora of serum biomarkers being studied, S100 calcium-binding protein B (S100B), neuron-specific enolase (NSE), interleukin-6, matrix metalloproteinase-9, asymmetric dimethylarginine, glial fibrillary acidic protein, and N-methyl-D-aspartic acid receptor subunit antibodies are of particular interest in AIS.7 The S100B is a calcium-binding peptide and is a useful neurobiochemical marker of brain damage, particularly in stroke, circulatory arrest, and traumatic brain injury.8 NSE, an isoenzyme of enolase, plays a key role in the formation of phosphoenolpyruvate from 2-phosphoglycerate, and is present in tissues of neuroectodermal origin. Cerebrospinal fluid (CSF) and serum S100B protein and NSE levels have been shown to predict outcomes in patients with acute brain ischemia and basal ganglia hemorrhage.9 10 Both of these biomarkers have been demonstrated as specific neurochemical markers of brain injury following stroke, and have been shown to correlate with infarct size as well as poststroke disability.11 12 However, majority of the studies on stroke biomarkers have been performed in the west. Some of these studies have reported a low sensitivity and specificity of NSE and S100B as biomarkers for differentiating AIS from stroke mimics.13 Only a handful of studies on stroke biomarkers have been conducted in India. Some of these studies from India studied only one biomarker (either NSE or S100B) at a time, some did not study correlation of these biomarkers with stroke severity/infarct size, and none of the prior Indian studies have attempted to find an association between NSE or S100B levels with various risk factors and clinical presentations among stroke patients.14 15 16 Hence, this study was conducted to evaluate the role of two biochemical markers, NSE and S100B in AIS, and sought to investigate their correlation with stroke severity in terms of infarct size among AIS patients. In addition, this study investigated whether there was any association between NSE or S100B levels with risk factors or clinical presentations of AIS patients.
Materials and Methods
Study Setting and Ethical Statement
This hospital-based observational cross-sectional study was conducted for a period of 18 months. Approval was taken from the institutional ethical committee before beginning recruitment of participants. Prior to enrollment, all subjects or their legally authorized representatives gave a written and informed bilingual consent.
Inclusion and Exclusion Criteria
Sixty stroke patients were recruited from both emergency as well as from inpatient services of a tertiary center in New Delhi, India. Patients > 18 years of age, presenting within 48 hours of onset of clinical signs and symptoms as per the World Health Organization definition of stroke,17 and radiological confirmation of AIS on noncontrast CT (NCCT) scan were included. Patients with history of any malignancy, neuroinflammatory disease, traumatic brain injury, intracranial bleed, prior neurodegenerative disorders, recent infection, transient ischemic attacks, and individuals refusing consent were excluded from the study.
Clinical Evaluation
Detailed general physical, systemic, and neurological examination findings were recorded for all patients using a structured pro forma. Traditional stroke risk factors such as age, hypertension, diabetes mellitus, dyslipidemia, history of smoking, alcohol intake, presence of carotid bruit, prior history of strokes, and history of rheumatic heart disease were recorded for all patients. Furthermore, stroke symptoms at the time of presentation such as loss of consciousness, seizures, bladder-bowel involvement, cranial nerve palsy, etc. were noted using the structured pro forma.
Investigations
Fasting venous blood sample was collected for all patients within 24 hours of presentation to the hospital. All routine hematological and biochemical assays (such as serum electrolytes, random blood glucose values, fasting lipid profile analysis, and liver and kidney functions) were performed immediately after separating the serum or plasma from the blood.
NSE and S100B Assays
After the initially separated plasma was used for routine analysis, the rest of the serum/plasma samples were stored at −80°C, until the batch was analyzed for levels of S100B and NSE with standard enzyme-linked immunosorbent assay kits. Batch analysis to measure serum S100B and NSE levels in the preserved samples was done using commercially available, monoclonal, two-site, sandwich immunoluminometric assay kits. The normal cutoff values for serum NSE and S100B were 0.625 ng/mL and 50 pg/mL, respectively.
Radiological Measurement of Infarct Volume and Blinding of Assessors
NCCT head was done within 48 hours of patient presenting with clinically suspected stroke using PHILIPS Brilliance 190P multidetector scan. The infarct size on NCCT head was evaluated by measuring the largest diameter in three planes. First, the largest diameter (a) of the infarct was measured. The second diameter (b) was calculated by measuring the largest diameter perpendicular to the first diameter. The third diameter (c) was calculated by multiplying the number of films in which the infarct was visible by the thickness of each slice. The final infarct volume was calculated using the formula abc/2.18 The radiologist assessing the infarct volume was not aware (blinded) of the serum values of NSE and S100B. Similarly, the individual assessing the serum values of NSE and S100B was not aware (blinded) of the patient's infarct volumes.
Statistical Analysis
The statistical analysis was performed using IBM SPSS (version 20) and STATA version 9. Mean ± standard deviation and range was used to describe continuous data wherever applicable. Categorical data was denoted in the form as percentages and proportions where applicable. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used for testing normality of continuous data. Pearson's correlation coefficient was applied to assess correlation between serum NSE and S100B levels and infarct volume (size). Infarct size was classified arbitrarily into three groups according to infarct volume (low, moderate, and large) and analysis of variance (ANOVA) was subsequently applied for comparing mean S100B and NSE levels in the three groups. We performed a multivariate logistic regression analysis to assess independent predictors of infarct size among stroke cases. Association between serum levels of S100B or NSE levels and clinical features among AIS cases was done by using the Mann–Whitney U test. A p-value of < 0.05 was taken as statistically significant.
Results
-
Baseline characteristics and mean serum levels of NSE and S100B in the study cohort
The mean age of the study cohort was 68 ± 13 years (range 26–86 years). Out of a total of 60 patients, 29 (48.3%) were males and 31 (51.7%) were females. Hypertension was present in 44 (73.3%) patients. Thirty patients (50%) were diabetic. History of tobacco smoking was found in 25 (41.7%) patients while 29 (48.3%) patients were noted to have dyslipidemia on fasting lipid profile analysis (Table 1).
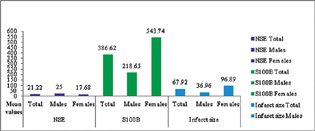
Mean S100B and NSE concentrations were 386.62 ± 68.79 pg/mL (7–1710 pg/mL) and 21.22 ± 2.24 ng/mL (5–100 ng/mL), respectively. Mean infarct size measured by CT scan was 67.92 ± 16.67 cm3 (range 0.48–700.21 cm3). Serum S100B level was significantly higher in females in comparison to males (p = 0.04). However, there was no significant association between gender and infarct size (Fig. 1).
-
Correlation between serum levels of S100B and NSE with infarct size
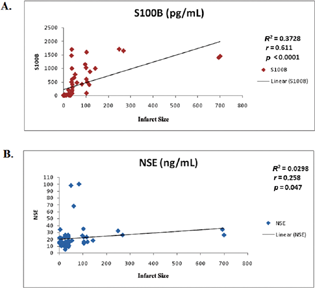
There was a positive correlation between S100B levels and infarct size and was found to be statistically significant (r = 0.611, p < 0.001). Similarly, serum NSE levels had a statistically significant positive correlation with infarct size (r = 0.258, p < 0.047). The relationship between infarct size and serum NSE and S100B levels is shown in Fig. 2A and B.
-
Association between serum biomarker levels with infarct volume (small/moderate/large)
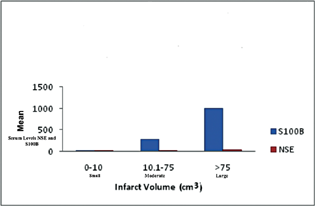
According to the volume of infarct on NCCT head, infarct size was arbitrarily classified into three groups: group 1–small infarcts (infarct volume of 0–10 mL), group 2–infarcts of moderate volumes (infarct volume of 10.1–75 mL), and group 3–large infarcts (infarct volume > 75 mL). On comparing the mean serum S100B and NSE levels in the three groups, it was found that larger infarcts had higher mean S100B and NSE levels (Fig. 3). Using ANOVA, the mean S100B and NSE levels in the three groups were compared, and it was found that there was a statistically significant difference between the three groups (p < 0.001) for S100B but not for NSE levels.
-
Association between infarct size, traditional stroke risk factors, and clinical presentations of stroke
We performed a multivariate logistic regression analysis taking infarct size as a dependent variable which showed that bladder/bowel involvement, prior history of stroke, and dyslipidemia were significantly associated with a larger infarct size (Table 2). On applying the Mann–Whitney U test, we found that only bladder-bowel involvement among stroke cases was associated with increased S100B and NSE levels (p-value (S100B) = 0.005, 95% confidence interval [CI] 712.16–1005.56; p-value (NSE) = 0.002, 95% CI (NSE) 30.56–44.80).
-
Fig. 1 Distribution of mean values of serum neuron-specific enolase (NSE), S100 calcium-binding protein B (S100B), and infarct size overall and among males and females. Bar diagram representing the distribution of mean values (y-axis) of S100B and NSE and infarct size (x-axis) overall, and among males and females in the study.
Fig. 1 Distribution of mean values of serum neuron-specific enolase (NSE), S100 calcium-binding protein B (S100B), and infarct size overall and among males and females. Bar diagram representing the distribution of mean values (y-axis) of S100B and NSE and infarct size (x-axis) overall, and among males and females in the study.
-
Fig. 2 (A and B) Scatter diagram representing the correlation between serum S100 calcium-binding protein B (S100B) (r = 0.611, p < 0.001) and neuron-specific enolase (NSE) (r = 0.258, p < 0.047) levels (y-axis) as compared with infarct volume (x-axis) in study subjects, respectively.
Fig. 2 (A and B) Scatter diagram representing the correlation between serum S100 calcium-binding protein B (S100B) (r = 0.611, p < 0.001) and neuron-specific enolase (NSE) (r = 0.258, p < 0.047) levels (y-axis) as compared with infarct volume (x-axis) in study subjects, respectively.
-
Fig. 3 Mean levels of S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) in the three study groups. Bar diagram representing the mean serum levels of S100B and NSE (y-axis) in the three study groups (x-axis): Group 1—small infarcts (0–10 mL volume), group 2—moderate volume infarcts (10.1–75 mL), and group 3—infarcts (volume > 75 mL).
Fig. 3 Mean levels of S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) in the three study groups. Bar diagram representing the mean serum levels of S100B and NSE (y-axis) in the three study groups (x-axis): Group 1—small infarcts (0–10 mL volume), group 2—moderate volume infarcts (10.1–75 mL), and group 3—infarcts (volume > 75 mL).
|
Parameters |
Values/N (%) |
|---|---|
|
Age (mean ± SD) y |
68 ± 13 |
|
Males |
29 (48.3%) |
|
Females |
31 (51.7%) |
|
Loss of consciousness at presentation |
24 (40%) |
|
Seizure at presentation |
6 (10%) |
|
Speech affection at presentation |
53 (88.3%) |
|
Bowel/bladder involvement at presentation |
9 (15%) |
|
Prior stroke at presentation |
8 (13.3%) |
|
Alcohol intake |
13 (21.7%) |
|
Rheumatic heart disease |
6 (10%) |
|
Other chronic medical illnesses |
7 (11.7%) |
|
Raised jugular venous pressure |
6 (10%) |
|
Carotid bruit |
19 (31.7%) |
|
Cranial nerve involvement |
52 (86.7%) |
|
Sensory system abnormality |
17 (28.3%) |
|
Diabetes mellitus |
30 (50%) |
|
Hypertension |
44 (73.3%) |
|
Smoking |
25 (41.7%) |
|
Dyslipidemia |
29 (48.3%) |
|
Infarct volume (mean ± SD) mL |
67.92 ± 16.67 |
|
S100B (mean ± SD) pg/mL |
386.62 ± 68.79 |
|
Serum neuron-specific enolase (mean ± SD) ng/mL |
21.22 ± 2.24 |
Abbreviation: SD, standard deviation.
|
Infarct size |
Coefficient |
Standard error |
T |
p-Value |
Lower limit 95% CI |
Upper limit 95%CI |
|---|---|---|---|---|---|---|
|
Gender |
–12.81 |
54.236 |
–0.240 |
0.815 |
–122.337 |
96.726 |
|
H/O loss of consciousness |
29.56 |
29.065 |
1.020 |
0.315 |
–29.138 |
88.259 |
|
H/O seizure |
43.97 |
46.132 |
0.950 |
0.346 |
–49.198 |
137.134 |
|
H/O speech affection |
53.93 |
55.662 |
0.970 |
0.338 |
–58.483 |
166.343 |
|
H/O bowel/bladder involvement |
146.58 |
42.885 |
3.420 |
0.001 a |
59.977 |
233.193 |
|
H/O smoking |
–0.96 |
56.521 |
–0.020 |
0.987 |
–115.102 |
113.192 |
|
H/O alcohol intake |
–18.01 |
38.114 |
–0.470 |
0.639 |
–94.978 |
58.966 |
|
H/O hypertension |
17.18 |
30.451 |
0.560 |
0.576 |
–44.319 |
78.673 |
|
H/O prior stroke |
82.65 |
39.179 |
2.110 |
0.041 a |
3.525 |
161.773 |
|
H/O diabetes |
67.16 |
40.061 |
1.680 |
0.101 |
–13.748 |
148.061 |
|
Carotid bruit |
12.23 |
32.368 |
0.380 |
0.707 |
–53.136 |
77.603 |
|
Cranial nerve involvement |
–16.82 |
67.071 |
–0.250 |
0.803 |
–152.271 |
118.633 |
|
Sensory abnormality |
62.42 |
32.438 |
1.920 |
0.061 |
–3.087 |
127.931 |
|
Dyslipidemia |
–85.45 |
39.744 |
–2.150 |
0.038 a |
–165.712 |
–5.183 |
Abbreviations: CI, confidence interval; H/O, history of.
Discussion
Stroke is a devastating neurological condition. The best strategy for reducing its burden is by limiting the extent of damage to the brain tissue, and by decreasing the risk of stroke-related death through early diagnosis and immediate therapeutic interventions. A biomarker which can be assessed via a rapid blood test would thus be very useful for diagnosis or risk stratification. The essential properties to consider in such blood-based biomarkers would be absence in the blood under normal circumstances, ease of measurement, rapidity of rise after the event, accuracy, reproducibility, cost, and low false positive or false negative results.19 20 NSE and S100B are two such biomarkers which can be of utility in diagnosing as well as predicting infarct size among ischemic stroke patient. NSE is the neuronal isoenzyme of enolase and under normal circumstances it is found in minute quantities in blood.21 S100B is an intracellular calcium-binding protein belonging to the EF-hand type calcium-binding protein family.22 S100B is predominantly found in astrocytes and NG2 cells (oligodendroglial precursor cells) and under normal circumstances it is not found in the blood or CSF.23 24 Thus, these two biomarkers are highly specific for neuronal or astrocytic damage and elevated blood levels have been found in stroke, neurodegenerative diseases, and traumatic brain injury.25 26
This cross-sectional observational study's primary objective was to investigate the relationship between NSE and S100B levels with ischemic stroke and to investigate their correlation with infarct size among AIS patients. We found that S100B levels were significantly higher among female stroke patients compared with their male counterparts. These findings may be due to the slightly higher proportion of females in our study (probably due to referral bias as our hospital predominantly caters to female patients) and an overall small sample size. Thus, larger studies would be required before any conclusions can be drawn.
Our results revealed that the mean serum S100B and NSE levels were elevated among ischemic stroke patients and a significant positive correlation was observed between these biomarkers and infarct size. These findings are in line with prior studies conducted from various parts of the world.12 27 28 In a meta-analysis of 13 case–control studies, serum NSE levels were significantly elevated among Asian and Caucasian acute cerebral infarction patients whereas serum S100B levels were elevated only among Asian stroke patients.29 However, the heterogeneity among the studies in this meta-analysis was quite high and further studies on the role of these biomarkers in stroke are still warranted.
Only two other studies have investigated the correlation of either serum NSE or S100B with infarct volume among Indian patients.14 16 The results of our study are in line with both the Indian studies.
In addition to prior studies, another objective (secondary) of our study was to investigate any association between various stroke risk factors and patient's clinical presentation with mean serum NSE, S100B levels, and infarct size. On logistic regression analysis, it was found that bladder/bowel involvement, prior history of stroke, and presence of dyslipidemia were independently associated with a larger infarct size. It is well known that the presence of dyslipidemia is a strong risk factor for atherosclerosis and would in turn result in increased stroke risk. Severe dyslipidemia would again predispose to greater atherosclerotic vascular occlusion and may thus result in larger infarct sizes. Our study also found that larger infarct sizes were independently associated with a prior history of stroke. This finding has been previously reported by Moroney et al who found that a major hemispheric stroke syndrome carried a 2.9 greater relative risk of recurrent stroke in the first 90 days.30
We noted that bladder and bowel involvement after stroke correlated with larger infarct size and increased S100B and NSE levels. In a study of 51 patients with hemispheric stroke, incontinence was present in patients with larger infarcts.31 Hence, larger infarcts with greater neuronal or glial cell loss would lead to greater release of S100B and NSE which may be a possible explanation for the findings in our study. However, the absolute number of stroke cases with bladder/bowel involvement was very small in our study (n = 9). Thus, studies with a larger sample size involving specific clinical presentations of stroke and their correlation with blood biomarkers levels could be an area of future interest and research.
Our study had limitations. This was a cross-sectional study without long-term patient follow-up. We did not have a control group and the overall sample size was relatively small. We did not study any correlation of these stroke biomarkers with long-term functional outcome (such as mortality or morbidity). Larger studies including a control group, along with the assessment of neuroanatomical region of stroke and their association with blood biomarkers could prove helpful for substantiating our results as the neuroanatomical region involved is an equally important predictor of stroke severity apart from the infarct size.
Conclusion
In conclusion, elevated serum levels of S100B protein and NSE had a significant positive correlation with infarct size in North Indian AIS subjects. In addition, bladder and bowel involvement, dyslipidemia, and prior history of stroke were associated with larger infarct size. Furthermore, the levels of NSE and S100B in the serum were higher among AIS patients presenting with bladder bowel involvement. In a developing country like ours, where there are logistic and economic concerns for availability and use of neuroimaging studies, biochemical markers could be of considerable value for diagnosis, predicting stroke severity, prognosis, and management of stroke patients.
Conflict of Interest
None declared.
Funding None.
References
- Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439-458.
- [Google Scholar]
- Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40(11):3646-3678.
- [Google Scholar]
- Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke. 2015;46(1):98-101.
- [Google Scholar]
- Diagnosis of DWI-negative acute ischemic stroke: a meta-analysis. Neurology. 2017;89(3):256-262.
- [Google Scholar]
- Genomic profiles for human peripheral blood T cells, B cells, natural killer cells, monocytes, and polymorphonuclear cells: comparisons to ischemic stroke, migraine, and Tourette syndrome. Genomics. 2006;87(6):693-703.
- [Google Scholar]
- Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ. 2016;2(1):28-47.
- [Google Scholar]
- The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21(4):134-140.
- [Google Scholar]
- Neuron-specific enolase and S 100B protein as predictors of outcome in ischaemic stroke. Neurol Neurochir Pol. 2010;44(5):459-463.
- [Google Scholar]
- Neuron-specific enolase, S100 calcium-binding protein B, and heat shock protein 70 levels in patients with intracranial hemorrhage. Medicine (Baltimore). 2015;94(45):e2007.
- [Google Scholar]
- The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci. 2005;12(5):542-547.
- [Google Scholar]
- The relationship of serum S100B levels with infarction size and clinical outcome in acute ischemic stroke patients. Noro Psikiyatri Arsivi. 2014;51(4):395-400.
- [Google Scholar]
- Serum neuron-specific enolase and S100 calcium binding protein B biomarker levels do not improve diagnosis of acute stroke. J R Coll Physicians Edinb. 2012;42(3):199-204.
- [Google Scholar]
- Correlation of serum S100 protein level with involvement of territory and size of lesion in acute ischemic stroke. Int J Adv Med Health Res. 2016;3:16-19.
- [Google Scholar]
- Role of blood biomarkers in differentiating ischemic stroke and intracerebral hemorrhage. Neurol India. 2020;68(4):824-829.
- [Google Scholar]
- Correlation between serum neuron specific enolase and functional neurological outcome in patients of acute ischemic stroke. Ann Indian Acad Neurol. 2013;16(4):504-508.
- [Google Scholar]
- Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113-130.
- [Google Scholar]
- Delayed increase in infarct volume after cerebral ischemia: correlations with thrombolytic treatment and clinical outcome. Stroke. 1999;30(3):502-507.
- [Google Scholar]
- Blood biomarkers in ischemic stroke: potential role and challenges in clinical practice and research. Crit Rev Clin Lab Sci. 2018;55(5):294-328.
- [Google Scholar]
- Stroke biomarkers in clinical practice: a critical appraisal. Neurochem Int. 2017;107:11-22.
- [Google Scholar]
- Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269-295.
- [Google Scholar]
- Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100b (beta beta) protein: Zn2+ regulates Ca2+ binding on S100b protein. J Biol Chem. 1986;261(18):8192-8203.
- [Google Scholar]
- Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci. 2004;27(4):453-465.
- [Google Scholar]
- NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58(11):1113-1124.
- [Google Scholar]
- A role of serum-based neuronal and glial markers as potential predictors for distinguishing severity and related outcomes in traumatic brain injury. J Korean Neurosurg Soc. 2015;58(2):93-100.
- [Google Scholar]
- Usefulness of S100B protein in neurological disorders. J Pak Med Assoc. 2011;61(3):276-281.
- [Google Scholar]
- Changes of protein S100B serum concentration during ischemic and hemorrhagic stroke in relation to the volume of stroke lesion [in Polish] Neurol Neurochir Pol. 2005;39(4):310-317.
- [Google Scholar]
- S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28(10):1956-1960.
- [Google Scholar]
- Elevated serum levels of NSE and S-100β correlate with increased risk of acute cerebral infarction in Asian populations. Med Sci Monit. 2015;21:1879-1888.
- [Google Scholar]
- Risk factors for early recurrence after ischemic stroke: the role of stroke syndrome and subtype. Stroke. 1998;29(10):2118-2124.
- [Google Scholar]
- Causes of urinary incontinence after acute hemispheric stroke. Stroke. 1993;24(3):378-382.
- [Google Scholar]







