Translate this page into:
Thiamine Deficiency and Neurological Symptoms in Patients with Hematological Cancer Receiving Chemotherapy: A Retrospective Analysis
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives Patients with hematological cancer receiving chemotherapy have a high risk of thiamine deficiency due to accelerated thiamine usage by tumor cells. Mild or severe thiamine deficiency can lead to varying degrees of neurological symptoms. We evaluated the relationship between thiamine deficiency and neurological symptoms, including mild or nonspecific symptoms, and the influence of chemotherapy on thiamine serum levels in patients with hematological cancer receiving chemotherapy.
Materials and Methods We retrospectively identified 42 patients diagnosed with hematological cancer at our hospital, using electronic medical records collected from March 2019 to March 2020. We evaluated the risk factors associated with neurological symptoms (mild-to-severe cognitive impairment, attention impairment, and mood or emotional disorder), the relationship between the presence of neurological symptoms and thiamine serum levels, and changes in thiamine serum levels after chemotherapy.
Results Thiamine deficiency was significantly associated with neurological symptoms. The thiamine serum levels in the group with neurological symptoms were significantly lower than those in the group without neurological symptoms. The Wilcoxon rank-sum test showed that thiamine serum levels after chemotherapy were significantly lower than those before administration of chemotherapy.
Conclusion Thiamine serum levels in patients with hematological cancer may be used as a reference to maintain neurological status during chemotherapy.
Keywords
thiamine deficiency
hematological
cancer
chemotherapy
neurological symptoms
Introduction
Thiamine deficiency induced by tumor cells, gastrointestinal disorders, and chemotherapy can develop in patients with cancer.1 2 3 In particular, thiamine deficiency can be more likely to occur in patients with hematological cancer, because of accelerated thiamine usage by tumor cells.4 5 6 Thiamine deficiency may lead to memory disorders,7 cognitive impairment,8 9 10 and mood disorders or depression.11 12 13 Most reports on thiamine deficiency have suggested a relationship between thiamine deficiency and severe neuropsychiatric disorders (e.g., Wernicke–Korsakoff syndrome10 14 15) in patients with various diseases. However, nonspecific symptoms such as weariness, headache, anorexia, sleep and mood disorders, poor coordination, and cognitive impairment related to thiamine deficiency, have been reported.16 Thiamine-related neurological symptoms may not be limited to only severe disease but also in nonspecific neurological symptoms caused by a mild deficiency in thiamine levels, and their symptoms can vary. However, the reports have been very limited and require further investigation.
Hematological and gastric cancers are the most common cancer types that lead to thiamine deficiency, because of decreased absorption and accelerated usage of thiamine.1 3 Although the number of patients was small, previous studies have shown that even mild deficiency in thiamine serum levels was associated with neurological symptoms (cognitive impairment, attention decline, and mood disorder) in patients with gastrointestinal cancer.17 18 19 However, there have been no reports on this in patients with hematological cancer. The relationship between mild-to-severe thiamine deficiency and neurological symptoms, including mild or nonspecific symptoms in patients with hematological cancer receiving chemotherapy, should be evaluated.
Furthermore, thiamine serum levels are not regularly evaluated in most hospitals.20 In clinical practice, thiamine serum levels are evaluated only for patients who develop severe neurological symptoms, suspected to be caused by thiamine deficiency. Additionally, serum thiamine is often analyzed at an outside laboratory, and results are returned after a few days. Hence, thiamine deficiency is often overlooked in practice. In fact, 80% of cases of Wernicke–Korsakoff syndrome were diagnosed after death.14
However, thiamine deficiency is not uncommon in patients with cancer.1 3 Cognitive impairment leads to a lack of adherence and self-care for chemotherapy-related adverse events,21 and mood or emotional disturbance and depression are injurious for cancer therapy. These previous studies suggested a relationship between neurological symptoms related to thiamine deficiency and absence of thiamine serum levels evaluation in patients with cancer. We believe that thiamine deficiency can develop more frequently and may lead to mild-to-severe neurological symptoms. Even if the neurological symptoms induced by thiamine deficiency were mild or nonspecific, these symptoms can be injurious for patients with hematological cancer receiving chemotherapy. Hence, an evaluation of thiamine serum levels at an early stage may be important.
The present study aimed at evaluating the relationship between thiamine deficiency and neurological symptoms in patients with hematological cancer receiving chemotherapy. In addition, we investigated changes in thiamine serum levels before and after administration of chemotherapy in chemotherapy-naïve patients, as a subset analysis, to evaluate the influence of chemotherapy on thiamine serum levels.
Materials and Methods
Study Design
This was a single-center, retrospective observational study. We enrolled patients diagnosed with hematological cancer who were admitted to the IMSUT Hospital, The Institute of Medical Science, The University of Tokyo (hereafter, our hospital) between March 2019 and March 2020. We only included patients with hematological cancer who had their thiamine serum levels measured. We performed a risk factor analysis in relation to neurological symptoms, including mild or nonspecific symptoms (cognitive impairment, attention impairment, and mood or emotional disorder). Thiamine serum levels were measured at the start of chemotherapy or from the middle course of chemotherapy and were continuously measured during the chemotherapy.
Regarding the definition of the presence of neurological symptoms, patients who had neurological symptoms, such as cognitive impairment, attention impairment, and mood disorder on their electronic medical records in which an incident report was filed or where treatment was interfered with, were considered to have neurological symptoms. The presence of neurological symptoms was recorded from episodes up to 7 days before measurements of thiamine serum levels. Furthermore, we investigated the relationship between serum thiamine levels and the occurrence of neurological symptoms. As a subgroup analysis, we evaluated the changes in thiamine serum levels before and after the administration of chemotherapy in chemotherapy-naïve patients. The cohort laboratory test results and clinical data were retrospectively collected from electronic medical records. We excluded patients with brain metastasis—those with a medical history of cerebrovascular accident or depression, or patients who were administered thiamine-containing agents prior to the start of chemotherapy. In accordance with the Japanese reference guidelines, serum thiamine levels < 24 ng/mL were considered to be low.
Statistical Analysis
We performed a multivariate analysis (logistic regression, including factors with p < 0.05 in the univariate analysis) to investigate the risk factors for neurological symptoms, including mild or nonspecific symptoms. The number of covariates included in the logistic model was limited due to the small number of events. In addition, we performed a comparative analysis using the Mann–Whitney U test of the thiamine serum levels between the patient groups with and without neurological symptoms, including mild or nonspecific symptoms. To evaluate thiamine serum levels before or after chemotherapy administration, the Wilcoxon rank-sum test was performed. The threshold for significance was set at p < 0.05. Statistical analyses were performed using EZR version 1.36 software (Saitama Medical Center, Jichi Medical University; Saitama, Japan).22
Results
Patient Characteristics
A total of 42 patients were enrolled in this retrospective study. No patient was excluded because of the exclusion criteria. The median age was 65 years, and the sex ratio was approximately 1:1. The most common type of cancer was non-Hodgkin lymphoma (29%). The division between lymphoma, myeloma, leukemia, and other types of hematological cancer was 33%, 19%, 36%, and 12%, respectively. Moreover, 98% of the patients did not have a history of alcohol consumption. According to the Eastern Cooperative Oncology Group (ECOG) Performance Status, all patients had a score of 0 (fully active, able to carry on all predisease performance without restriction). Although the baseline median laboratory values did not significantly deviate from the reference values, thiamine serum levels were lower than the reference values, and some patients were affected with hepatic impairment, renal impairment, and high levels of inflammation. Some patients had abnormal laboratory values of the blood cell system because of the disease (Table 1).
|
Median age, years (range) |
65 (20–83) |
|
|---|---|---|
|
Gender (male), n (%) |
23 (55) |
|
|
Cancer type, n (%) |
Non-Hodgkin lymphoma |
12 (29) |
|
Hodgkin lymphoma |
2 (4.8) |
|
|
Multiple Myeloma |
8 (20) |
|
|
Adult T-cell leukemia |
7 (17) |
|
|
Acute myeloid leukemia |
5 (12) |
|
|
Acute lymphocytic leukemia |
1 (2.4) |
|
|
Chronic myelogenous leukemia |
2 (4.8) |
|
|
Myelodysplastic syndromes |
1 (2.4) |
|
|
Langerhans cell histiocytosis |
4 (9.5) |
|
|
Drinking history (no), n (%) |
41 (98) |
|
|
ECOG performance status, n (%) |
0 (%) |
42 (100) |
|
1 (%) |
0 (0) |
|
|
2 (%) |
0 (0) |
|
|
Median (range) |
||
|
Body mass index (kg/m2) |
23.3 (15.8–28.7) |
|
|
Laboratory parameter |
||
|
Aspartate aminotransferase (IU/L) |
23.5 (10–434) |
|
|
Alanine aminotransferase (IU/L) |
17.5 (6–87) |
|
|
Total bilirubin (mg/dl) |
0.5 (0.2–1.5) |
|
|
Serum creatinine (mg/dL) |
0.78 (0.42–1.66) |
|
|
White blood cell count (102/μL) |
36.6 (6.6–357.2) |
|
|
Red blood cell count (104/μL) |
352 (208–560) |
|
|
Hemoglobin concentration (g/dL) |
10.5 (6.5–17.0) |
|
|
Serum albumin (g/dL) |
3.6 (1.9–4.4) |
|
|
Serum total protein (g/dL) |
6.7 (4.9–11.7) |
|
|
C-reactive protein (mg/dL) |
0.29 (0.01–22.28) |
|
|
Serum vitamin B12 level (pg/mL) |
- (92- > 1500) |
|
|
Serum folic acid level (ng/mL) |
- (1.7- > 22) |
|
|
Serum thiamine level (ng/mL) |
22 (11–96) |
|
|
History of pretreatment, n (%) |
24 (57.1) |
|
|
Neurological symptoms (yes), n (%) |
||
|
Cognitive impairment (%) |
2 (4.8) |
|
|
Attention decline (%) |
11 (26) |
|
|
Mood disorder (%) |
13 (31) |
|
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Multivariate Analysis (Including Factors with p < 0.05 in the Univariate Analysis)
A logistic regression analysis revealed that a decrease in thiamine serum levels was the only statistically significant factor associated with neurological symptoms, including mild or nonspecific symptoms (p = 0.001, odds ratio = 0.015, 95% CI: 0.001–0.180). The explanatory variables selected were p < 0.05 in the univariate and multivariate analyses. Since age > 65 years (p = 0.90), serum albumin ≤ 4.1 mg/dL (p = 0.81), serum vitamin B12 level < 180 pg/mL (p = 0.11), serum folic acid level < 4.0 ng/mL (p = 0.06), serum natrium level < 139 mEq/L (p = 0.13), and sex (p = 0.85) all had a p > 0.05 in the univariate analysis, they were not included in the explanatory variables in the multivariate analysis (Table 2).
|
95% CI for OR |
||||
|---|---|---|---|---|
|
p-Value |
OR |
Lower |
Upper |
|
|
Serum thiamine (< 24 ng/mL) |
0.001 |
0.015 |
0.001 |
0.180 |
|
Serum calcium (< 8.8 mg/dL) |
0.156 |
3.830 |
0.599 |
24.50 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Note: Factors with p < 0.05 in the univariate analysis were used as explanatory variables to perform multivariate analysis.
Relationship between the Presence of Neurological Symptoms, Including Mild or Nonspecific Symptoms and Thiamine Serum Levels
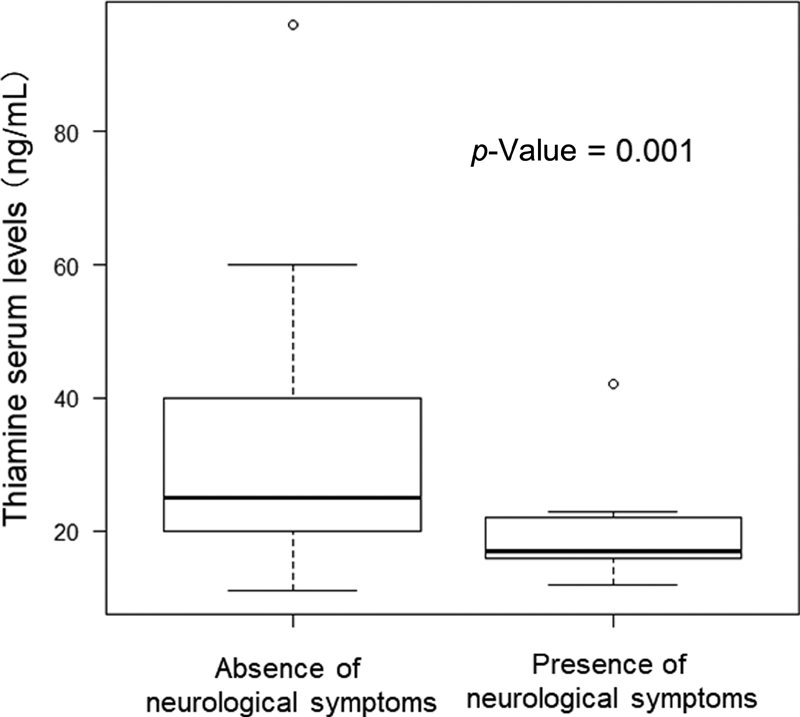
The relationship between the presence of neurological symptoms and thiamine serum levels is shown in Fig. 1. The group with neurological symptoms had significantly lower serum thiamine levels (19.5 ± 5.4 ng/mL) than the group without neurological symptoms (31.9 ± 14.2 ng/mL, Mann–Whitney U test, p = 0.001) (Fig. 1).
-
Fig. 1 Relationship between the presence/absence of neurological symptoms and thiamine serum levels. Cognitive impairment, attention impairment, and mood disorder are classified as neurological symptoms. The Mann–Whitney U test was used for analysis. The thiamine serum levels were 31.9 ± 14.2 (absence of neurological symptoms) and 19.5 ± 5.4 (presence of neurological symptoms).
Fig. 1 Relationship between the presence/absence of neurological symptoms and thiamine serum levels. Cognitive impairment, attention impairment, and mood disorder are classified as neurological symptoms. The Mann–Whitney U test was used for analysis. The thiamine serum levels were 31.9 ± 14.2 (absence of neurological symptoms) and 19.5 ± 5.4 (presence of neurological symptoms).
Changes in Thiamine Serum Levels after Chemotherapy
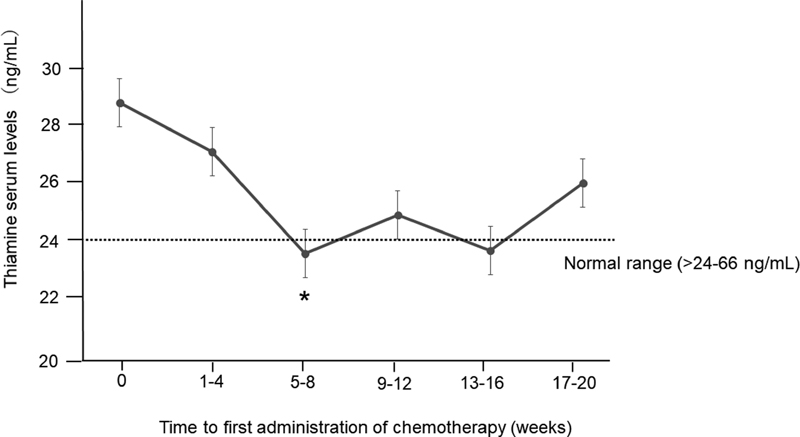
Subanalysis was performed in chemotherapy-naive patients. After the administration of chemotherapy, thiamine serum levels gradually declined, with the lowest levels at 5 to 8 weeks (23.5 ± 7.6 ng/mL, p = 0.035 vs. 0-week, Wilcoxon rank-sum test) (Fig. 2).
-
Fig. 2 Changes in thiamine serum levels after the administration of chemotherapy. Subanalysis in chemotherapy-naive patients via the Wilcoxon rank-sum test. *p = 0.035 versus 0 weeks.
Fig. 2 Changes in thiamine serum levels after the administration of chemotherapy. Subanalysis in chemotherapy-naive patients via the Wilcoxon rank-sum test. *p = 0.035 versus 0 weeks.
Discussion
This study investigated the relationship between mild-to-severe thiamine deficiency and neurological symptoms, including mild or nonspecific symptoms and changes in thiamine serum levels before and after administration of chemotherapy in patients with hematological cancer. The results showed that a decrease in thiamine serum levels was associated with neurological symptoms. Regarding the relationship between the presence of neurological symptoms and thiamine serum levels, the thiamine serum levels in the group with neurological symptoms were significantly lower than those in the group without neurological symptoms. In addition, thiamine serum levels declined significantly after administration of chemotherapy. None of the patients were suspected to have neurological disorders induced by electrolyte abnormalities, dehydration, and anemia, because the patients' laboratory data during the study period were normal.
Previous studies have shown that thiamine deficiency is related to various neurological symptoms.10 23 24 However, thiamine-related neurological symptoms do not all present as serious symptoms, and mild or nonspecific symptoms may occur. If the patients are not treated for mild or subclinical thiamine deficiency, severe neurological symptoms may develop, which can be life-threatening. Although almost all reports have suggested an association between thiamine deficiency and severe neurological symptoms (for example, Wernicke–Korsakoff syndrome or depression),13 14 15 25 26 27 few reports have reported a relationship between thiamine deficiency, including mild deficiency, and neurological symptoms, including mild or nonspecific symptoms. Mechanisms involved in developing mild or nonspecific neurological symptoms, induced by mild thiamine deficiency, can be the same as those for severe neurological symptoms induced by severe thiamine deficiency. In particular, studies are required to examine the influence of gastrointestinal cancer and hematological cancer as risk factors for thiamine deficiency. Previous studies have shown that mild deficiency in thiamine serum levels was associated with neurological symptoms (cognitive impairment, attention decline, and mood disorder) in patients with gastrointestinal cancer.17 18 19 However, similar studies with patients with hematological cancer are required.
In cells, thiamine is a coenzyme for dehydrogenation in the glucose/amino acid metabolism pathway and plays an essential role in energy metabolism.28 29 A lack of thiamine leads to failure of the Krebs cycle. The accumulation of ketones in the body and the production of free radicals by the above mechanisms can adversely affect neurons in the brain.28 Because of their high-cell metabolism, patients with hematological cancer are likely to have rapid progress of the above mechanisms than patients with other cancer types.5 6 Therefore, patients with hematological cancer may have a high risk of thiamine deficiency.
As expected, the logistic regression analysis showed that the decrease in thiamine serum levels was a statistically significant factor associated with neurological symptoms, including mild or nonspecific symptoms in this study. Other factors may have been associated with neurological symptoms: (1) serum vitamin B12 level30 and serum folic acid level31 can be related to neurological symptoms, (2) abnormalities in serum calcium and sodium levels can be associated with various neurological symptoms, (3) women are more likely to develop depressive symptoms compared with males,32 and (4) elderly people tend to have a lower cognitive function. However, these factors were not detected as significant factors; consequently, only the decrease in thiamine serum levels was considered to be a factor associated with neurological symptoms in this study. This result shows a strong correlation between serum thiamine levels and neurological symptoms in patients with hematological cancer receiving chemotherapy. In addition, the thiamine serum levels in the group with neurological symptoms were significantly lower than those in the group without neurological symptoms. The results of our study were concordant with those of a previous study on the relationship between severe neurological symptoms and thiamine deficiency. Regarding the changes in thiamine serum levels before and after the administration of chemotherapy, increased thiamine consumption33 in tumor cells and chemotherapy may further increase thiamine consumption as a result of enhanced cellular metabolism.
This study showed that neurological symptoms, including mild or nonspecific symptoms, were associated with thiamine deficiency in patients with hematological cancer receiving chemotherapy. Before progression to severe condition, mild or nonspecific symptoms associated with mild thiamine deficiency may develop. If thiamine serum levels are not evaluated, these mild symptoms may progress to more serious conditions. In patients with hematological cancer, thiamine deficiency may develop in the early stage. Therefore, based on these results, we suggest that evaluating thiamine serum levels in patients with early-stage hematological cancer may be important to better maintain neurological status during the therapy period. However, because some patients had subclinical thiamine deficiency, the presence of thiamine deficiency did not necessarily mean that they would develop neurological symptoms, and thiamine serum levels should be taken as one of the reference values.
Limitations
Several limitations of this study should be acknowledged. The limitations of the present study are as follows: (1) The number of cases is limited, which decreases the statistical power of the logistic regression analysis. (2) Assessments of cognitive decline, mood, or emotional disorder by objective scoring could not be performed. (3) We did not evaluate brain imaging findings (CT and MRI scans). (4) Further long-term investigations on the differences in thiamine deficiency according to anticancer drug types are warranted. (5) The analysis of neurological adverse events was limited to cognitive impairment, attention decline, and mood or emotional disorder, while we did not examine other neurological adverse events such as peripheral nerve disorders. Further studies with a larger number of cases are required. (6) In this study, we evaluated patients with good general status, but the same analysis should be conducted in patients with poor performance status in the future.
Conclusions
In summary, we performed a retrospective observational study to evaluate the relationship between neurological symptoms, including mild or nonspecific symptoms, and thiamine deficiency in patients with hematological cancer receiving chemotherapy. Neurological symptoms were associated with thiamine deficiency, and patients with neurological symptoms had lower serum thiamine levels.
Acknowledgment
The originality regarding the association between thiamine deficiency and neurological symptoms was first reported by Dr. Takeo Yasu. The authors are grateful to Dr. Yasu for suggesting the topic of this article.
Conflict of Interest
None declared.
Funding None.
References
- Thiamine deficiency in patients with B-chronic lymphocytic leukaemia: a pilot study. Postgrad Med J. 2001;77(911):582-585.
- [Google Scholar]
- Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol. 2011;18(13):3711-3717.
- [Google Scholar]
- Thiamin status of patients treated with drug combinations containing 5-fluorouracil. Eur J Cancer. 1980;16(8):1041-1045.
- [Google Scholar]
- Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
- [Google Scholar]
- Wernicke encephalopathy complicating lymphoma therapy: case report and literature review. South Med J. 2007;100(7):717-719.
- [Google Scholar]
- Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86(12):1362-1368.
- [Google Scholar]
- Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine deficiency at early pre-pathological lesion stage. Neurobiol Dis. 2008;29(2):176-185.
- [Google Scholar]
- Nonalcoholic thiamine-related encephalopathy (Wernicke-Korsakoff syndrome) among inpatients with cancer: a series of 18 cases. Psychosomatics. 2016;57(1):71-81.
- [Google Scholar]
- Thiamine supplementation mood and cognitive functioning. Psychopharmacology (Berl). 1997;129(1):66-71.
- [Google Scholar]
- Influence of thiamin supplementation on the health and general well-being of an elderly Irish population with marginal thiamin deficiency. J Gerontol. 1991;46(1):M16-M22.
- [Google Scholar]
- Adjuvant thiamine improved standard treatment in patients with major depressive disorder: results from a randomized, double-blind, and placebo-controlled clinical trial. Eur Arch Psychiatry Clin Neurosci. 2016;266(8):695-702.
- [Google Scholar]
- Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
- [Google Scholar]
- Palliative treatment of thiamine-related encephalopathy (Wernicke's encephalopathy) in cancer: a case series and review of the literature. Palliat Support Care. 2015;13(5):1241-1249.
- [Google Scholar]
- Vitamins.Clinical Chemistry: Principles, Procedures, Correlations. Philadelphia, New York: Lippincott; 1996. p. :607-609. In: eds.
- [Google Scholar]
- Thiamine deficiency as a possible cofactor causing cognitive dysfunction in a patient with end-stage gastric cancer. Int J Clin Pharmacol Ther. 2019;57(8):416-419.
- [Google Scholar]
- Potential thiamine deficiency and neurological symptoms in patients receiving chemotherapy for gastrointestinal cancer. Int J Clin Pharmacol Ther. 2020;58(3):139-145.
- [Google Scholar]
- Potential thiamine deficiency in elderly patients with gastrointestinal cancer undergoing chemotherapy. Int J Clin Pharmacol Ther. 2020;58(3):174-176.
- [Google Scholar]
- Biomarkers for detecting thiamine deficiency–improving confidence and taking a comprehensive history are also important. Alcohol. 2010;45(2):213.
- [Google Scholar]
- Geriatric Oncology Research in the Cooperative Groups: A Report of a SIOG Special Meeting. J Geriatr Oncol. 2010;1(1):40-44.
- [Google Scholar]
- Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
- [Google Scholar]
- High rate of thiamine deficiency among inpatients with cancer referred for psychiatric consultation: results of a single site prevalence study. Psychooncology. 2017;26(9):1384-1389.
- [Google Scholar]
- Early detection and successful treatment of Wernicke encephalopathy in a patient with advanced carcinoma of the external genitalia during chemotherapy. Palliat Support Care. 2016;14(3):302-306.
- [Google Scholar]
- Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr Rev. 2016;74(5):281-300.
- [Google Scholar]
- Wernicke encephalopathy without delirium in patients with cancer. Palliat Support Care. 2018;16(1):118-121.
- [Google Scholar]
- Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430(1):3-43.
- [Google Scholar]
- Neurological symptoms of vitamin B12 deficiency: analysis of pediatric patients. Acta Clin Croat. 2019;58(2):295-302.
- [Google Scholar]
- Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154(12):1641-1647.
- [Google Scholar]
- Glucose and acetate utilization by hyperplastic, alveolar nodule outgrowths and adenocarcinomas of mouse mammary gland. Cancer Res. 1971;31(5):527-537.
- [Google Scholar]






