Translate this page into:
Clinical Profile of Cognitive Decline in Patients with Parkinson's Disease, Progressive Supranuclear Palsy, and Multiple System Atrophy
Address for correspondence: Dr. Sulena, H. No. 12, Gali No. 1 Guru Nanak Colony, Faridkot - 151 203, Punjab, India. E-mail: sulenasingh@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
There are very less data on the comparison between the cognitive profile in Parkinson's disease (PD) and Parkinson’s-plus groups, especially in India.
Aims:
The aim of this study is to compare the cognitive profile across PD, progressive supranuclear palsy (PSP), and multiple system atrophy (MSA) groups and compare them using Mini–Mental State Examination (MMSE), frontal assessment battery (FAB), and verbal fluency tests.
Settings and Design:
This was a cross-sectional study.
Materials and Methods:
MMSE, FAB, and verbal fluency tests were administered in a total of 73 patients constituting 22 patients in MSA, 26 patients in PD, and 25 patients in PSP group, respectively. Twenty-six participants both age- and gender-matched were enrolled in control group.
Statistical Analysis:
Statistical analysis was done using SPSS Version 20.0. Descriptive statistics were done to find out the mean and standard deviation of different variables. ANOVA was done for followed by post hoc Bonferroni test to assess the cognitive function in three groups.
Results:
ANOVA showed that there is a significant difference for MMSE scores (P = 0.038) being worse scores for PSP and maximum for MSA. A significant difference was found for FAB scores within three groups. There is a significant difference for FAB scores (P = 0.00003) being worse scores for PSP and highest scores obtained for PD. All the subtests of FAB test differed significantly except motor programming across MSA, PSP, and PD groups.
Conclusions:
Our data suggest that global cognitive impairment and executive dysfunction are worst in PSP among the three groups. Patients with MSA had significant cognitive decline as opposed to previous experience. FAB scores and verbal fluency tests are good tests to assess cognitive impairment in these diseases. Subsets of FAB score have significant differences but cannot help differentiating conclusively between these three diseases.
Keywords
Cognitive profile
Parkinson disease
Parkinson-plus syndrome
INTRODUCTION
Parkinsonism is a syndrome characterized by tremor at rest, rigidity, bradykinesia, loss of postural reflexes, flexed posture, and freezing. Parkinson disease (PD) is the most common cause of Parkinsonism in patients referred to specialized movement disorder clinics. The second most common group of Parkinsonism, being categorized clinically as Parkinsonism-plus syndromes depending on associated neurological features.
The spectrum of nonmotor features in Parkinson and Parkinsonism-plus syndromes (progressive supranuclear palsy [PSP], multiple system atrophy [MSA]) is broad and often missed in clinical practice. Cognitive disturbances are one of the important nonmotor features which have as much impact on the quality of life of a patient with Parkinsonism as the motor symptoms.[1] The cognitive impairment has long been ignored, and with the advancement in management of these patients and increased life expectancy, the cognitive decline is becoming apparent and needs to be addressed. Foltynie et al. evidenced that 36% of those in an incident cohort of PD patients had evidence of cognitive impairment.[2]
PSP is characterized by supranuclear ophthalmoparesis and Parkinsonism and was first described by Steele et al. in 1964.[3] Mild dementia was noticed in early stages of the disease in their original monograph. Despite greater awareness in recent years, cognitive decline in PSP remain undiagnosed or misdiagnosed for much of their disease duration.
MSA was described by Graham and Oppenheimer in 1969, as a syndrome characterized clinically by Parkinsonism and dysautonomia.[4] Cognitive impairment was considered a nonsupporting feature, and the first diagnostic criteria considered dementia as an exclusion criterion for the diagnosis of MSA.[5] However, in the past few years, cognitive impairment has been found to be a frequent feature in MSA based on evidence from qualitative neuropsychological assessment. Dementia in MSA is now reported in 14%–16% of cases.[67]
There is dearth of literature regarding cognitive profile in PD and Parkinsonism-plus syndromes in India. In one of the studies from India, comparing neuropsychological functions in PD and Parkinson-plus syndromes, global, and frontal dysfunction were worst in PSP as compared to PD and MSA, and severity of cognitive dysfunction in these diseases was related to the distribution and extent of pathological changes affecting the striatofrontal circuits.[8]
PD is differentiated from other forms of Parkinsonism, including MSA and PSP by defining clinical criteria's as no reliable diagnostic biomarkers have been described yet. The differences in the cognitive profile of these three diseases can help in differentiating and understanding the basic pathological differences among these diseases. This information may be helpful in guiding physician, patients, and their families in the planning for long-term rehabilitative care.
MATERIALS AND METHODS
This was a cross-sectional study conducted in a tertiary care center in Rajasthan in the rural population. This study was done in a time span of 1 year. Routine admissions were monitored to neurology ward, and patients of PSP, PD, and MSA were enrolled with clinical diagnosis of PD according to the UKPD diagnostic criteria,[9] PSP using the National Institute for Neurological Disorders and Society for PSP criteria[10] and MSA using consensus diagnostic criteria.[11] Patients gave informed consent, and this study was approved by the ethics committee of the hospital.
The proforma consisted of three sections where the first section contained epidemiological information about the patient's age, sex, education, and residence. The second section contained information about the present symptoms and duration of diseases. The third section consisted of neurological examination.
The screening of cognition was assessed using Mini–Mental State Examination (MMSE). MMSE scores below 24 were taken as indicating cognitive impairment. Detailed neuropsychological evaluation was done, and patients were assessed for frontal, parietal, temporal, and occipital lobe dysfunction. Patients where then specifically assessed for frontal lobe dysfunction using frontal assessment battery (FAB score) and verbal fluency test.
FAB score consists of six subtests: (i) Similarities (conceptualization); (ii) lexical fluency (mental flexibility); (iii) motor series (programming); (iv) conflicting instructions (sensitivity to interference); (v) go–no go (inhibition control); and (vi) prehension behavior (environmental autonomy). Each subtest is rated from 3 to 0, with the total score, therefore, ranging from 18 to 0. Performance on the test correlates well with other conventional tests of executive function.[12] FAB scores below 12 were taken as indicating cognitive impairment.
The verbal fluency test is a short test of verbal functioning. It consists of tests for fluency (initial letter fluency[13] starting with the letter “P” and category fluency for animals-time 1 min).[14] The participant's score in each task was the number of unique correct words.
The mean scores were calculated and compared among three diseases and also with age-matched controls. The control group consisted of healthy age, gender- and education-matched individuals, and a total of twenty participants were taken as control. Patients were excluded if the history of any other neurological disease, brain injury, substance abuse, major depressive or psychotic disorder, delirium, stroke, transient ischemic attacks, brain tumors or brain surgery, and individuals with uncorrected visual or auditory defects.
The aim of the present study is to study the difference in the cognitive profile of patients with PD, MSA, and PSP.
Statistical analysis: means and standard deviations were determined for each group using SPSS Version 20.0. Armonk, NY: IBM Corp and comparison of the MMSE and FAB scores of participants and controls and then separately of all three groups were done using ANOVA and Bonferroni method. The same procedure of comparison was followed for the subset scores also.
RESULTS
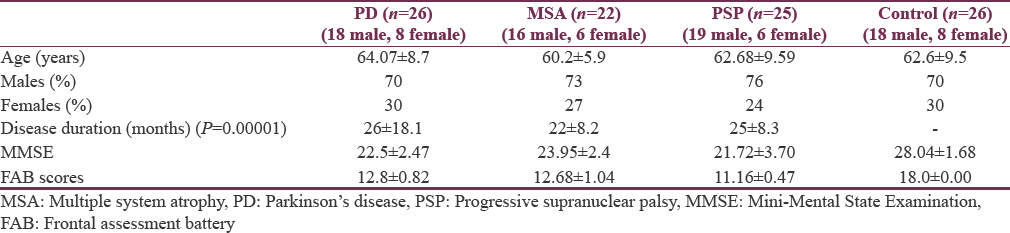
A total of 73 patients were enrolled in pathological group constituting 22 patients in MSA, 26 patients in PD, and 25 patients in PSP group, respectively. Twenty-six participants both age- and gender-matched were enrolled in control group. The mean age of experimental group and control group was 62.4 ± 8.37 years and 61.05 ± 7.07 years, respectively. The gender distribution for three subgroups (diagnosis group) and control group is given in Table 1. It was observed that males were more in every experimental group compared to females though not clinically significant within and across the groups (P = 0.276). The demographics are shown in Table 1.
Majority of the patients were from lower education group. Forty percent of MSA, 60% of PSP, and 42.3% of PD were 10th passed. 22.7% of MSA, 4.0% of PSP, and 15.4% of PD patients were 12th passed. Almost less than one-fourth of patients in each group were graduates and very few <15% were seen as postgraduates.
When compared within subgroups (MSA, PD, and PSP), it was seen disease duration was significantly different (P = 0.00001) within the groups. Presentation of cognitive impairment was earliest in PSP (22 ± 8.2 months) and PD (25 ± 8.3 months) as compared to MSA (26 ± 18.1 months).
Table 2 shows P values are significantly different across three neurological conditions when compared with control group. ANOVA is done to study within group effect between different pathological groups which shows that there is a significant difference for MMSE scores (P = 0.038) being worse scores for PSP and maximum for MSA. Post hoc Bonferroni test shows that there is a significant difference for MMSE scores when MSA group compared with PSP but not with PD group.
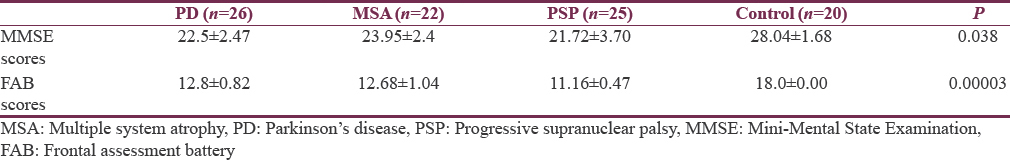
A significant difference was found for FAB scores within three groups. The highest scores obtained for PD and least for PSP. ANOVA test showed that there is a significant difference for FAB scores (P = 0.00003) between different pathological groups, being worse scores for PSP (11.16 ± 0.47). Post hoc Bonferroni test shows that there is a significant difference for FAB scores when MSA group compared with PSP but not with PD group and PD group varies significantly from PSP. ANOVA test showed that all the subtests of FAB test differed significantly across MSA, PSP, and PD groups except motor programming.
Post hoc Bonferroni test analysis shows that there is a significant difference for conceptualization scores between MSA and PSP groups as shown in Table 3. Similarly, between PSP and PD and PSP varies from MSA. In addition, post hoc Bonferroni test analysis for mental flexibility shows that MSA varies significantly with PD and PSP. Motor programming scores did not differ significantly on post hoc analysis. Post hoc Bonferroni test analysis shows that there is a significant difference for sensitivity to interference among MSA and PD groups and MSA and PSP groups. Similarly, inhibitory control scores vary significantly on post hoc Bonferroni analysis of MSA from PD and PSP groups. In environmental autonomy, only PSP scores show a significant variation from MSA.
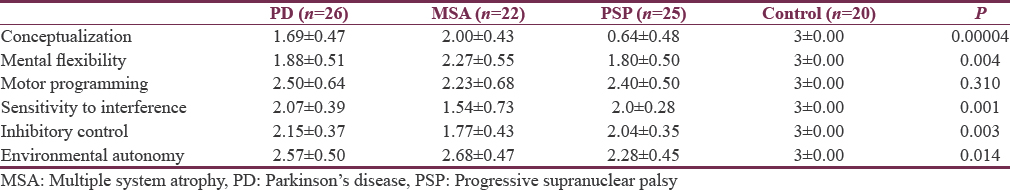
Table 4 shows P value is significantly different across the experimental and control group for both the fluency tests. Post hoc Bonferroni test analysis shows that there is a significant difference for semantic fluency test among MSA (14.77 ± 1.74) and PD (12.07 ± 2.36) groups and MSA and PSP (16.28 ± 0.73) groups. Similarly, semantic fluency test scores vary significantly for PD scores from MSA and PSP groups.

For letter fluency test, post hoc Bonferroni test analysis shows that there is a significant difference among MSA (13.95 ± 0.78) and PD (10.96 ± 0.958) groups and MSA and PSP (11.84 ± 2.82) groups. Similarly, post hoc Bonferroni test analysis for letter fluency test starting from P shows a significant variation for MSA scores from PD and PSP groups.
DISCUSSION
The cognitive decline in Parkinson and Parkinson-plus syndrome varies from mild impairment of memory and executive functions to full-blown dementia.[15] Executive dysfunction is a prominent feature in PD and Parkinson-plus syndrome.[16] Reductions in activity of frontostriatal neural circuitry are related to cognitive impairments in these diseases.[17]
Disease duration and severity of cognitive impairment are only partially related, reflecting different rates of disease progression. Some patients develop cognitive impairment and later dementia within few years after onset of PD, whereas others may develop dementia twenty or more years after disease onset.[18] In our study, patients with cognitive impairment were significantly older in Parkinson disease than other two groups as compared to the previous study, where patients with PSP were older than MSA.[7] When compared within subgroups (MSA, PD, and PSP), it was seen disease duration was significantly different within the groups. Presentation of cognitive impairment was earliest (P = 0.00001) in PSP (22 ± 8.2 months) and as compared to PD (25 ± 8.3 months) and MSA (26 ± 18.1 months) as shown in Table 1.
The prevalence of PD and Parkinson-plus syndromes is lower in India as compared with Caucasian populations.[192021] Only a few studies have been done on cognitive profile of patients with atypical parkinsonism in world as well as India.[815]
The assessment of cognitive function was done using MMSE, FAB score, and verbal fluency tests across three groups. This study showed that mean MMSE scores were worst in PSP (21.72 ± 3.70) followed by PD (22.5 ± 2.47 and MSA 23.95 ± 2.4). In a similar study, the MMSE ranges were 17–30 with PSP, 21–30 for patients with MSA, and 24–30 for patients with PD.[22] The poorer scores of MMSE in PSP can be attributed to poor mobility and slowness of movement, occurring in nearly 70% of cases.[23] Our study shows that mean MMSE scores vary significantly for MSA from PSP but not with PD. This goes well in concordance to another study from India[8] which stated MMSE was significantly worse in PSP as compared to PD, however, no significant difference was seen between MSA and PD.
However, MMSE cannot assess well the executive function which is affected most in these patients. Executive functions have been defined as capacities that “enable a person to engage successfully in independent, purposive, and self-serving behavior,” and encompass cognitive processes such as initiation, planning, purposive action, self-monitoring, self-regulation, volition, inhibition, and flexibility.[24]
In a previous cross-sectional, multicenter study from the European MSA group, it was seen that general cognitive decline as assessed by MMSE was uncommon (2 out of 61 patients had score <24). In contrast, frontal lobe-related functions (as measured by FAB) were impaired in 41% of patients, with abstract reasoning and sustained attention less compromised in same group.[25] This pattern was similar to control group of twenty patients with PD (matched for disease duration and age at onset).
Our study showed significant difference was found for FAB scores within three experimental groups. The highest scores were obtained for PD and least for PSP. There was a significant difference for FAB scores when MSA group was compared with PSP but not with PD group and PD group varies significantly from PSP. Our results were similar to another study, where 71.8% of the PSP group scored below 15 on FAB score while 62.0% scored below 14. In the MSA-P and C group, the figures were 42.3% and 31.8%, respectively, and on the MMSE, 33.6% of the PSP group scored below 25.[7]
When comparing cognitive profiles of synucleinopathies, it is interesting to note that cognitive impairment is more pronounced in dementia with Lewy bodies and only mild in PD, whereas MSA patients present an intermediate profile similar to our study (MMSE in PSP 21.72 ± 3.70, PD 22.5 ± 2.47, MSA 23.95 ± 2.4).[26]
When comparing MSA and PSP using FAB, MMSE, and Mattis Dementia Rating Scale, the profile observed in MSA and PSP were similar, albeit more severe in patients with PSP.[7]
Another study suggested that the FAB score discriminates executive dysfunction in bradykinetic rigid syndromes.[27] They noticed that FAB scores were significantly lower in PSP than in MSA or PD (P = 0.02 and P < 0.001) and were also found to be significantly lower in MSA than in PD (P = 0.047) similar to our study. While 82% of the PSP group had FAB scores of <15, such scores were recorded in only 36% of the MSA and 8% of the PD groups.[27] Another study showed executive dysfunction is quite prominent in patients with PSP where 16 out of 20 PSP patients scored below 15 on FAB.[28]
Using data from the PRIAMO study where cognitive impairment was defined as a Mini–Mental Status Evaluation score ≤23.8 and FAB score ≤13.48. Total FAB scores and single FAB items were lower in Parkinson's-plus syndromes versus PD.[29]
Our study showed that all the subtests of FAB test differed significantly except motor programming. There is a significant difference for conceptualization scores between MSA and PSP groups, between PSP and PD, and PSP varies from MSA significantly.
Our study showed that there is a significant difference for sensitivity to interference among MSA and PD groups and MSA and PSP groups. Similarly, inhibitory control scores vary significantly in MSA from PD and PSP groups. In environmental autonomy, only PSP scores show a significant variation from MSA.
In our study, mental flexibility shows that MSA varies significantly from PD and PSP. However, motor programming scores did not differ significantly on post hoc analysis among three experimental groups where as in another study,[27] motor series subscores and lexical fluency of the FAB discriminated 70% of the PSP, MSA, and PD patients. A step-wise regression analysis revealed that across the groups, among the variables that correlated with FAB scores, alternating semantic fluency accounted for 80% of FAB score variance.
In our study, there is a significant different across the experimental and control group for both the fluency tests. Mean semantic fluency scores were significantly low in PD as compared to MSA followed by PSP. Post hoc Bonferroni test analysis shows that there is a significant difference for semantic fluency test among MSA and PD groups and MSA and PSP groups. Similarly, semantic fluency test scores vary significantly for PD scores from MSA and PSP groups. It was noted in another study,[8] no statistically significant difference between MSA and PD (95% CI: −3.08, 1.4; uncorrected P = 0.3, corrected P = 1), whereas the PSP group performed worse than MSA group (95% CI: 1.6, 6.08; corrected and uncorrected P < 0.001) and PD group (95% CI: −6.92,-2.44; corrected and uncorrected P < 0.001).
In the present study, letter fluency test shows that there is a significant difference among MSA and PD groups and MSA and PSP groups. In a study from India, no statistically significant difference in the scores between the MSA and PD groups (95% CI: −1.67, 3.11; corrected P = 1, uncorrected P = 0.5) on the initial letter fluency task, whereas PSP group fared significantly worse than MSA group (95% CI: 2.21, 6.99; corrected and uncorrected P < 0.001) and PD group.[8] Similarly, a large meta-analysis has shown that verbal fluency impairment is more pronounced than that seen in PD nondemented patients, and also, semantic fluency seems to be more compromised than phonemic fluency.[29]
In our study, global and frontal cognitive deficits were worst in PSP than other two groups. Cognitive function in PSP is seen to be affected in up to 60% of cases, with prominent deficits in attention and executive function, with verbal fluency being severely affected, as well as deficits in both verbal and nonverbal memory with a relative preservation of recognition.[7]
In another study, patients with PSP showed greater impairment in both phonemic and semantic fluency than patients with PD or MSA. Over 90% of patients with PSP were correctly classified. Patients with PD and MSA were correctly classified in over 70% of cases.[29] These results suggest that verbal fluency tasks may be sensitive measures in the differential diagnosis of PD, MSA, and PSP.
Limitation of study
-
While the MMSE has been used traditionally to screen for cognitive deficits, it often fails to detect early cognitive decline because of its ceiling effect
-
The foremost weakness is that major motor disability or communication problems can interfere with performance of the test
-
Pathological confirmation for the diagnosis was not available in our cases.
CONCLUSIONS
Our data suggest that global cognitive impairment and executive dysfunction are worst in PSP among the three groups. Patients with MSA had significant cognitive decline as opposed to previous experience. FAB scores and verbal fluency tests are good tests to assess cognitive impairment in these diseases. Subsets of FAB score have significant differences but cannot help differentiating conclusively between these three diseases. A fuller understanding of cognitive function in PD, MSA, and PSP needs large-scale prospective studies with pathological confirmation of diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The cognitive ability of an incident cohort of Parkinson's patients in the UK. the camPaIGN study. Brain. 2004;127:550-60.
- [Google Scholar]
- Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333-59.
- [Google Scholar]
- Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969;32:28-34.
- [Google Scholar]
- Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94-8.
- [Google Scholar]
- What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;68:434-40.
- [Google Scholar]
- Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133:2382-93.
- [Google Scholar]
- Neuropsychological functions in progressive supranuclear palsy, multiple system atrophy and Parkinson's disease. Neurol India. 2006;54:268-72.
- [Google Scholar]
- Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-4.
- [Google Scholar]
- Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-richardson-olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology. 1996;47:1-9.
- [Google Scholar]
- Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670-6.
- [Google Scholar]
- Missile Wounds of the Brain: A Study of Psychological Deficits. London: Oxford University Press; 1969.
- Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53-60.
- [Google Scholar]
- Neuropsychological pattern of striatonigral degeneration: Comparison with Parkinson's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1995;58:174-9.
- [Google Scholar]
- Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200-13.
- [Google Scholar]
- Beyond and below the cortex: The contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J Neurol Neurosurg Psychiatry. 2014;85:371-8.
- [Google Scholar]
- Epidemiological study of neurological disorders in a rural population of eastern India. J Indian Med Assoc. 2003;101:299-300, 302.
- [Google Scholar]
- Prevalence of neurological disorders in Bangalore, India: A community-based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23:261-8.
- [Google Scholar]
- A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163-72.
- [Google Scholar]
- Clinical features and natural history of progressive supranuclear palsy: A clinical cohort study. Neurology. 2003;60:910-6.
- [Google Scholar]
- Functions of the frontal lobes: Relation to executive functions. J Int Neuropsychol Soc. 2011;17:759-65.
- [Google Scholar]
- A cross-sectional multicenter study of cognitive and behavioural features in multiple system atrophy patients of the parkinsonian and cerebellar type. J Neural Transm (Vienna). 2013;120:613-8.
- [Google Scholar]
- Factors influencing the cognitive function in patients with multiple system atrophy. Mov Disord. 2010;25:2891-2.
- [Google Scholar]
- Can the frontal assessment battery (FAB) differentiate bradykinetic rigid syndromes? relation of the FAB to formal neuropsychological testing. Neurocase. 2005;11:274-82.
- [Google Scholar]
- Utility of Frontal Assessment Battery in detection of neuropsychological dysfunction in Richardson variant of progressive supranuclear palsy. Neurol Neurochir Pol. 2015;49:36-40.
- [Google Scholar]
- Frontal assessment battery scores and non-motor symptoms in parkinsonian disorders. Neurol Sci. 2012;33:585-93.
- [Google Scholar]
- Verbal fluency deficits in Parkinson's disease: A meta-analysis. J Int Neuropsychol Soc. 2004;10:608-22.
- [Google Scholar]






