Translate this page into:
Nature of Parkinsonian features in multiple system atrophy
*Corresponding author: Ruchika Tandon, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. rtlib161080@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pradhan S, Tandon R. Nature of Parkinsonian features in multiple system atrophy. J Neurosci Rural Pract. 2024;15:211-6. doi: 10.25259/JNRP_445_2023
Abstract
Objectives:
For this observational study, we evaluated the clinical profile of Parkinsonian features in multiple system atrophy (MSA), as there is no clarity about the specifics of these features in this disease compared to progressive supranuclear palsy (PSP) and Parkinson’s disease (PD).
Materials and Methods:
Here, we selected 57 patients with MSA based on standard criteria and grouped them into two categories – Parkinsonian variant of MSA (MSA-P) and cerebellar variant of MSA (MSA-C). However, researchers did not distinguish among patients based on the nature of extrapyramidal syndrome or levodopa responsiveness. Then, we examined the patients at the time of their first visit to outpatient clinics or indoor wards and recorded and analyzed the specific extrapyramidal features or their variations.
Results:
The extrapyramidal features including levodopa responsiveness were highly variable among MSA-C as well as MSA-P patients. A subset of patients presented with features resembling PSP (symmetry [56.1%], axial rigidity [52.6%], backward falls [28.1%], and down-gaze restriction [17.5%]), while others presented with features resembling PD (asymmetry [43.9%], tremors [71.9%], and peripheral rigidity [40.4%]). After grouping patients based on predominant extrapyramidal features, 36.8% of patients had PD-like, 19.3% had PSP-like, and 43.9 % had mixed presentation. Moreover, 86% of patients had a perceptible levodopa response, including a sustained response for more than six months in 64% of patients.
Conclusion:
Extrapyramidal features in MSA patients may be PD-like, PSP-like, or mixed. Moreover, an initial presentation resembling PSP or PD may be deceptive and one must follow it up for MSA.
Keywords
Multiple system atrophy
Parkinsonian variant of multiple system atrophy
Progressive supranuclear palsy
Parkinson’s disease
Parkinson’s disease-like presentation
Progressive supranuclear palsy-like presentation
INTRODUCTION
Multiple system atrophy (MSA) is a sporadic progressive neurodegenerative disorder comprising Parkinsonian features, cerebellar features, corticospinal dysfunction, and autonomic features.[1-4] MSA patients often come to the clinical attention due to Parkinsonian features.
However, the clinical profile of Parkinsonian features is not as clear in MSA as in some other neurodegenerative syndromes such as progressive supranuclear palsy (PSP) or Parkinson’s disease (PD). With time and validation now, there is a fair amount of clarity in Parkinsonian features to distinguish between PSP and PD. However, the literature considers some Parkinsonian features such as asymmetry, tremors, peripheral rigidity, and forward falls to be the typical features of PD,[5-10] while it does hold some others such as symmetry, axial rigidity, backward falls, and down-gaze restriction to be the typical features of PSP.[11-16]
In this paper, we studied Parkinsonian features in MSA to see if there is any specific pattern, which could be useful in the early diagnosis of MSA.
MATERIALS AND METHODS
In this observational study, the researchers studied patients of MSA in the Department of Neurology of the institute with an objective to study the type of Parkinsonian features in them.
The patients
The study included the MSA patients admitted to the wards and also those visiting the outdoors of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India between August 2017 and August 2022, and we selected patients with MSA based on the standard criteria (“The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy”)[1] and grouped them into two categories – Parkinsonian variant of MSA (MSA-P) and cerebellar variant of MSA (MSA-C).
However, we did not distinguish among patients at the time of selection based on the nature of extrapyramidal syndrome or levodopa responsiveness.
Exclusion criteria for cases included PD patients, patients of Parkinson-plus syndrome other than MSA, and patients not giving consent for the study.
Procedure
At the time of the patient’s first visit to the outpatient clinic or indoor wards, we took a history of present and past illness and performed a detailed clinical examination. Thereafter, the authors recorded and analyzed specific extrapyramidal features or their variations. They also applied the Unified Parkinson’s Disease Rating Scale at the time of the patient’s first visit and then two weeks after initiating levodopa therapy to look for initial response and thereafter six months later to look for a sustained response. Furthermore, the clinicians performed a magnetic resonance imaging (MRI) of the patients using 3T-MRI and took blood samples for blood tests (blood counts, liver, renal, and thyroid function tests, vitamin B12, and folic acid levels) and excluded from the study all those who had any of the abnormalities in the above-mentioned blood tests, as these could mimic some of the features of parkinsonism or dementia. Thereafter, we classified these patients into MSA-P and MSA-C.
Time line
4½ years-recruitment and data collection; 6 months-data analysis, writing, and revision of manuscript.
Statistics
Sample size calculation
The prevalence of MSA is 3.4–4.9/100,000 population[17] and the population of our city is estimated to be around 3.7 million.[18]
Hence, the number of people living with MSA in and around our city is around 160. Hence, if we calculate the sample size for a 10% margin of error, 95% confidence interval, and population proportion of 50%, we get a sample size of 61. In this study, we recruited 57 patients, after screening around 70.
Statistical analysis
The investigators calculated the mean, median, standard deviation, and range for different demographic parameters and frequency and percentage for the clinical characteristics. For calculating P-values for differences in different parameters in MSA-P and MSA-C groups, we applied the Chi-square tests and compiled the statistics using SPSS version 20.
The Institutional Ethics Committee of SGPGI, Lucknow approved the study of these patients.
RESULTS
Of all 57 cases, the mean age: 60.09 ± 10.216 years, 15 females and 42 males, 18 (31.6%) had MSA-C and 39 (68.4%) had MSA-P. The mean age at the onset of the disease was 55.95 ± 10.793 years. Table 1 depicts the demographic characteristics of the MSA-P and MSA-C patients. Figure 1 illustrates the clinical and imaging features of our MSA patients.
| Demographic and clinical characteristics | Frequency and percentage/Mean±SD in MSA patients n (%) | Frequency and percentage/Mean±SD in MSA-P patients n (%) |
Frequency and percentage/Mean±SD in MSA-C patients n (%) | P-value of difference between MSA-P and MSA-C | Chi-square value |
|---|---|---|---|---|---|
| Demographic features | |||||
| Age | 60.09±10.216 | 60.67±10.027 | 58.83±10.799 | 0.710 | 23.446 |
| Age at onset | 55.95±10.793 | 56.46±10.748 | 54.83±11.116 | 0.204 | 35.016 |
| Sex | |||||
| Males | 42 (73.7) | 30 (76.9) | 12 (66.7) | 0.414 | 0.668 |
| Females | 15 (26.3) | 9 (23.1) | 6 (33.3) | 0.414 | 0.668 |
| Clinical characteristics | |||||
| Parkinsonian features | |||||
| Back stiffness | 50 (87.7) | 36 (92.3) | 14 (77.8) | 0.120 | 2.414 |
| Neck stiffness | 48 (84.2) | 34 (87.2) | 14 (77.8) | 0.366 | 0.819 |
| Arm and leg stiffness | 51 (89.5) | 37 (94.9) | 14 (77.8) | 0.051 | 3.821 |
| Slowness of movements | 54 (94.7) | 39 (100) | 15 (83.3) | 0.009 | 6.861 |
| Tremors | 41 (71.9) | 29 (74.4) | 12 (66.7) | 0.548 | 0.361 |
| Pill rolling tremors | 12 (21.1) | 10 (25.6) | 2 (11.1) | 0.211 | 1.564 |
| Masking of face | 32 (56.1) | 28 (71.8) | 4 (22.2) | <0.001 | 12.292 |
| Change in handwriting | 48 (84.2) | 32 (82.1) | 16 (88.9) | 0.510 | 0.433 |
| Freezing | 25 (43.9) | 18 (46.2) | 7 (38.9) | 0.607 | 0.264 |
| Forward falls | 19 (33.3) | 13 (33.3) | 6 (33.3) | 1.000 | 0.000 |
| Backward falls | 16 (28.1) | 13 (33.3) | 3 (16.7) | 0.193 | 1.694 |
| Bradykinesia | 53 (93) | 39 (100) | 14 (77.8) | 0.002 | 9.321 |
| Truncal rigidity | 53 (93.0) | 36 (92.3) | 17 (94.4) | 0.769 | 0.086 |
| Neck rigidity | 49 (86.0) | 34 (87.2) | 15 (83.3) | 0.698 | 0.151 |
| Peripheral rigidity | 55 (96.5) | 38 (97.4) | 17 (94.4) | 0.568 | 0.326 |
| Cerebellar features | |||||
| Upper limb incoordination | 50 (87.7) | 33 (84.6) | 17 (94.4) | 0.293 | 1.105 |
| Lower limb incoordination | 51 (89.5) | 34 (87.2) | 17 (94.4) | 0.406 | 0.690 |
| Gait ataxia | 44 (77.2) | 27 (69.2) | 17 (94.4) | 0.035 | 4.441 |
| Nystagmus | 33 (57.9) | 21 (53.8) | 12 (66.7) | 0.362 | 0.830 |
| Miscellaneous | |||||
| Dysarthria | 33 (57.9) | 24 (61.5) | 9 (50) | 0.412 | 0.673 |
| Dysphagia | 12 (21.1) | 8 (20.5) | 4 (22.2) | 0.883 | 0.022 |
| Staring | 5 (8.8) | 4 (10.3) | 1 (5.6) | 0.560 | 0.340 |
| Snoring | 17 (29.8) | 11 (28.2) | 6 (33.3) | 0.694 | 0.155 |
| Pseudobulbar affect | 9 (15.8) | 4 (10.3) | 5 (27.8) | 0.092 | 2.844 |
| Abnormal saccades | 33 (57.9) | 23 (59) | 10 (55.6) | 0.808 | 0.059 |
| Broken pursuit | 34 (59.6) | 25 (64.1) | 9 (50) | 0.313 | 1.018 |
| Upgaze restriction | 38 (68.4) | 29 (74.4) | 10 (55.6) | 0.156 | 2.015 |
| Downgaze restriction | 11 (19.3) | 7 (17.9) | 4 (22.2) | 0.704 | 0.144 |
| Lateral gaze restriction | 19 (33.3) | 15 (38.5) | 4 (22.2) | 0.227 | 1.462 |
| Exaggerated jaw jerk | 15 (26.3) | 8 (20.5) | 7 (38.9) | 0.143 | 2.145 |
| Exaggerated gag reflex | 14 (24.6) | 8 (20.5) | 6 (33.3) | 0.296 | 1.093 |
| Spasticity | 31 (54.4) | 21 (53.8) | 10 (55.6) | 0.904 | 0.015 |
| Levodopa responsiveness | 49 (86) | 36 (92.3) | 13 (72.2) | 0.042 | 4.118 |
| Amantadine responsiveness | 26 (45.6) | 18 (46.2) | 8 (44.4) | 0.904 | 0.015 |
MSA: Multiple system atrophy, MSA-P: Parkinsonian variant, MSA-C: Cerebellar variant, SD: Standard deviation
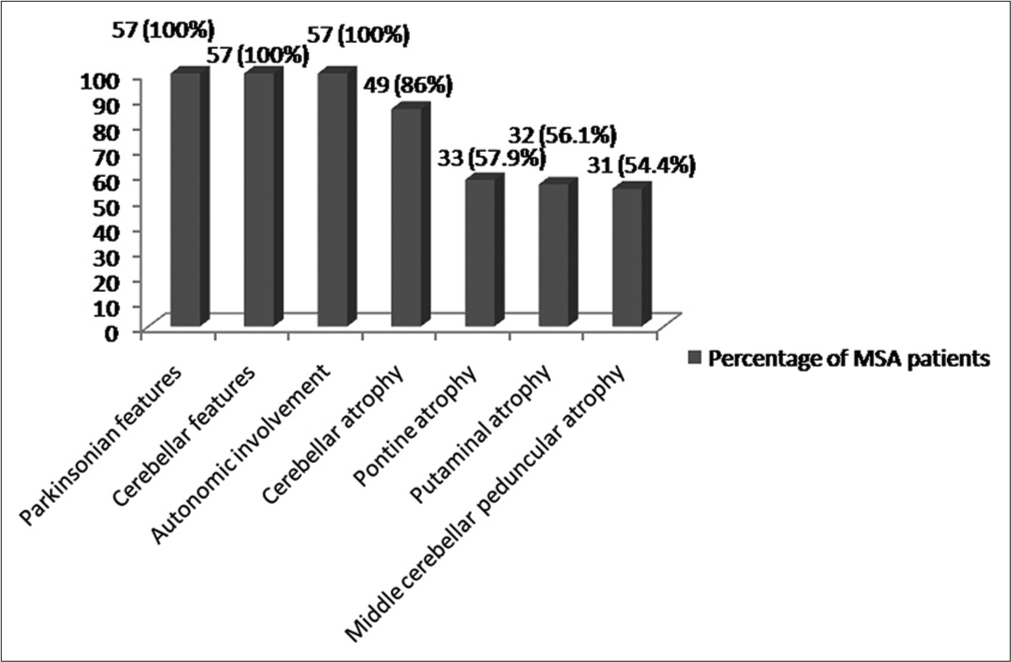
- Percentage of multiple system atrophy (MSA) patients having different clinical and imaging features
Although 86% of the MSA patients had an initial response to levodopa, 64% of them sustained this responsiveness to levodopa. Furthermore, amantadine is effective in around half of all patients. Table 1 demonstrates different clinical features found in MSA patients and in the MSA-P and MSA-C groups, and Table 2 depicts the frequency of features mimicking PSP and PD in MSA patients.
| S. No. | Clinical features | Frequency in MSA patients n(%) | Frequency in MSA-P patients n(%) | Frequency in MSA-C patients n(%) | P-value of difference between MSA-P and MSA-C | Chi-square value |
|---|---|---|---|---|---|---|
| 1. | Features mimicking PSP | |||||
| a. | Symmetrical rigidity | 32 (56.1) | 24 (61.5) | 8 (44.4) | 0.277 | 1.462 |
| b. | Axial rigidity more | 30 (52.6) | 21 (53.8) | 9 (50) | 0.787 | 0.073 |
| c. | Backward falls | 16 (28.1) | 13 (33.3) | 3 (16.7) | 0.193 | 1.694 |
| d. | Downgaze restriction | 11 (19.3) | 7 (17.9) | 4 (22.2) | 0.704 | 0.144 |
| e. | Upgaze restriction | 38 (68.4) | 29 (74.4) | 10 (55.6) | 0.156 | 2.015 |
| f. | Abnormal saccades | 33 (57.9) | 23 (59) | 10 (55.6) | 0.808 | 0.059 |
| g. | Abnormal pursuit | 34 (59.6) | 25 (64.1) | 9 (50) | 0.313 | 1.018 |
| 2. | Features mimicking PD | |||||
| a. | Peripheral rigidity more | 23 (40.4) | 14 (35.9) | 9 (50) | 0.631 | 0.230 |
| b. | Tremors | 41 (71.9) | 29 (74.4) | 12 (66.7) | 0.548 | 0.361 |
| c. | Asymmetrical rigidity | 25 (43.9) | 15 (38.5) | 10 (55.6) | 0.227 | 1.462 |
| d. | Forward falls | 19 (33.3) | 13 (33.3) | 6 (33.3) | 1.000 | 0.000 |
PSP: Progressive supranuclear palsy, MSA: Multiple system atrophy, MSA-P: Parkinsonian variant of MSA, MSA-C: Cerebellar variant of MSA, PD: Parkinson’s disease . MSA, MSA-P, and MSA-C patients having different types of extrapyramidal features
After grouping all 57 MSA (39 MSA-P and 18 MSA-C) patients on the basis of predominant extrapyramidal features, Figure 2 shows the number and percentage of those having PD-like (at least two of asymmetry, tremors, forward falls, and peripheral rigidity), PSP-like (at least two of symmetry, axial rigidity, backward falls, and gaze restriction), and a mixed presentation (P = 0.073).
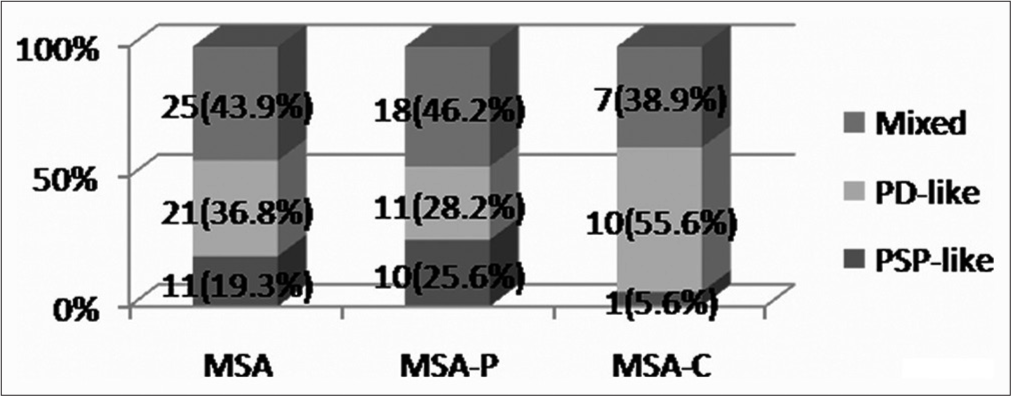
- Frequency and percentage of multiple system atrophy (MSA), parkinsonian variant of MSA (MSA-P), cerebellar variant of MSA (MSA-C). patients having different types of extrapyramidal features
Based on history, of all 57 MSA patients, 31 (54.39%) (22 MSA-P and 9 MSA-C) presented initially with pure Parkinsonian symptoms for a variable period before the appearance of any other symptom to suggest MSA (autonomic or cerebellar).
DISCUSSION
Apart from autonomic features, cerebellar features, and corticospinal dysfunction, the Parkinsonian features are an important part of MSA.[1-4] As there is a lot of disparity in the clinical extrapyramidal features between PD and PSP, it becomes important to understand the nature of these features in MSA as well. In the present study, among the clinical features mimicking PSP, 56.1% of all MSA patients had symmetry. Quite like our study, previous studies have also shown symmetry in around 60% of MSA patients.[19,20] Furthermore, previous researchers have shown axial rigidity to be more common in MSA.[20] In our study also, more than half of all MSA patients had an axial rigidity, which is consistent with the previous studies. Moreover, extraocular movement disorder is an important feature in the diagnosis of PSP and helps in differentiating between MSA and PD at an early stage.[21] However, in our study, quite a large number of patients of MSA suffered from an extraocular movement disorder in the form of downgaze restriction, upgaze restriction, abnormal saccadic eye movements, and abnormal smooth pursuit. Hence, these features may not be specific to PSP, and a large number of MSA patients may as well have these features. MSA patients may have falls.[20,22-24] However, we do not know exactly how many patients have falls in the backward direction like PSP and how many of them fall in the forward direction like PD patients. In this study, around one-third of all patients fell in the backward direction, which is a feature resembling PSP, and the forward falls were as frequent.
Among the clinical features mimicking PD, more than one-third of all MSA patients had an asymmetry. Some studies have shown asymmetry in MSA, but the reports are few.[25]
Tremor, however, is a known feature of MSA and may occur in as high as 80% of all MSA patients.[26] Our study also demonstrated tremors in >70% of the MSA patients, though only one-fifth of all patients had the typical pill-rolling tremors. Furthermore, around half of all MSA patients in the present study had a predominant peripheral rigidity, which experts consider to be a very typical feature of PD.
In all, 86% of MSA patients responded initially to levodopa, and the previous studies have shown variable response to levodopa in MSA patients.[20,27,28]
Hence, the initial presentation in some of the MSA patients was PD-like and in some others PSP-like. However, the highest proportion of them had a mixed type of presentation, and one must consider this fact while diagnosing MSA patients at an earlier stage.
Slowness of movements was the most frequent symptom in MSA patients, and peripheral rigidity was the most frequent sign. Among MSA-P patients, the most frequent symptom was slowness of movements and the most frequent sign was bradykinesia, while in MSA-C patients, the most frequent symptom was change in handwriting and the most frequent signs were peripheral rigidity, upper limb incoordination, lower limb incoordination, and truncal rigidity. In the past also, the studies reported slowness of movements and bradykinesia to be the most frequent clinical features of MSA, similar to our observation.[29] In MSA-P patients, also the results depicting bradykinesia to be the most frequent sign are similar to the previous observations, while in MSA-C, though we expected incoordination, a very high number of individuals had rigidity as well, which was contrary to our expectations.[29]
The only features that were significantly different in MSA-P and MSA-C groups were slowness of movements, masking of the face, and bradykinesia, which were more frequent among MSA-P patients and gait ataxia, which was more common in MSA-C patients, and these findings were consistent with the earlier knowledge.[30] Although not the primary aim of this study, we found that cerebellar atrophy was the most frequent imaging finding in our MSA patients, the others being pontine atrophy, putaminal atrophy, and middle cerebellar peduncular atrophy. According to past research, putaminal atrophy, hyperintense rim, cerebellar atrophy, the “hot cross bun” sign, and middle cerebellar peduncle hyperintensity are frequent in MSA. The previous researchers have found out decrease in glucose metabolism in the parietal area for PD, in the bilateral putamen for MSA-P, and in the bilateral cerebellum for MSA-C in 18F-fluorodeoxyglucose-positron emission tomography/computed tomography, and therefore, such techniques can differentiate between MSA and PD.[31]
The limitation of this study is that this is a single-center study, and the scientific community will benefit if we get data from other regions as well in the future.
Since more than half of all MSA patients presented initially with pure Parkinsonian symptoms before the appearance of any other symptoms suggestive of MSA (cerebellar or autonomic), hence, one must keep in mind this fact and follow up all the patients, who initially present as PSP or PD for the appearance of any other symptoms or signs suggestive of MSA, as the early clinical features may be deceptive. We can generalize these results to that of the population of at least our country considering the fact that our institute caters to a number of people from all parts of India.
CONCLUSION
The extrapyramidal features of MSA patients may resemble those of PD or PSP or they may have characteristics of both. Also, one must follow all the patients with a PD-like or PSP-like presentation to look for MSA.
Author’s contributions
SP: Concept, design, the definition of intellectual content. RT: Literature search and clinical studies, data acquisition, data analysis, and statistical analysis. SP and RT: Manuscript preparation. SP and RT: Manuscript editing and manuscript review.
Ethical approval
Approved by the Institutional Ethics Committee at SGPGI, Lucknow, number Approval number 2017-194-IMP-113, dated 21-02-2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov Disord. 2022;37:1131-48.
- [CrossRef] [PubMed] [Google Scholar]
- Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry. 2012;83:453-9.
- [CrossRef] [PubMed] [Google Scholar]
- The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: Clinicopathological correlations. Brain. 2004;127:2657-71.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history of multiple system atrophy: A prospective European cohort study. Lancet Neurol. 2013;12:264-74.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation of Parkinsonism-predominant multiple system atrophy from idiopathic Parkinson's disease Using 3T susceptibility-weighted MR imaging, focusing on putaminal change and lesion asymmetry. AJNR Am J Neuroradiol. 2015;36:2227-34.
- [CrossRef] [PubMed] [Google Scholar]
- Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson's disease. Parkinsonism Relat Disord. 2015;21:590-6.
- [CrossRef] [PubMed] [Google Scholar]
- Is reduced arm and leg swing in Parkinson's disease associated with rigidity or bradykinesia? J Neurol Sci. 2014;341:32-5.
- [CrossRef] [PubMed] [Google Scholar]
- Slow down and concentrate: Time for a paradigm shift in fall prevention among people with Parkinson's disease? Parkinsons Dis. 2013;2013:704237.
- [CrossRef] [PubMed] [Google Scholar]
- Fall events described by people with Parkinson's disease: Implications for clinical interviewing and the research agenda. Physiother Res Int. 1999;4:190-200.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive supranuclear palsy: Progression and survival. J Neurol. 2016;263:380-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of progressive supranuclear palsy in the society for progressive supranuclear palsy brain bank. Mov Disord. 2003;18:1018-26.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive supranuclear palsy: Clinical presentation and rehabilitation of two patients. Arch Phys Med Rehabil. 1993;74:537-9.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive supra-nuclear palsy: Frequency of cardinal extrapyramidal features at first presentation. Postgrad Med J. 2015;91:274-7.
- [CrossRef] [PubMed] [Google Scholar]
- Census. 2021. Available from: https://census-2021.co.in/lucknow-population-2021 [Last accessed on 2021 Oct 16]
- [Google Scholar]
- Clinical characteristics of multiple system atrophy in Serbian population. Vojnosanit Pregl. 2006;63:861-6.
- [CrossRef] [PubMed] [Google Scholar]
- Parkinsonism in multiple system atrophy: Natural history, severity (UPDRS-III), and disability assessment compared with Parkinson's disease. Mov Disord. 2002;17:701-9.
- [CrossRef] [PubMed] [Google Scholar]
- PSP as distinguished from CBD, MSA-P, and PD by clinical and imaging differences at an early stage. Intern Med. 2011;50:2775-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362-72.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of resistance training on balance and functional ability of a patient with multiple system atrophy. J Geriatr Phys Ther. 2008;31:79-83.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of falls and fractures in bradykinetic rigid syndromes: A retrospective study. J Neurol Neurosurg Psychiatry. 2006;77:468-73.
- [CrossRef] [PubMed] [Google Scholar]
- Markedly asymmetric presentation in multiple system atrophy. Parkinsonism Relat Disord. 2013;19:901-5.
- [CrossRef] [PubMed] [Google Scholar]
- Tremor in multiple system atrophy-a review. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-165-4252-1.
- [CrossRef] [Google Scholar]
- Response to L-DOPA in multiple system atrophy. Clin Neuropharmacol. 1993;16:139-44.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacodynamics of a low subacute levodopa dose helps distinguish between multiple system atrophy with predominant parkinsonism and Parkinson's disease. J Neurol. 2016;263:250-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebellar and Parkinsonian phenotypes in multiple system atrophy: Similarities, differences and survival. J Neural Transm (Vienna). 2014;121:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple system atrophy-cerebellar type: Clinical picture and treatment of often-overlooked disorder. Cureus. 2020;12:e10741.
- [CrossRef] [Google Scholar]
- Clinical features, MRI, and 18F-FDG-PET in the differential diagnosis of Parkinson's disease from multiple system atrophy. Brain Behav. 2020;10:e01827.
- [CrossRef] [PubMed] [Google Scholar]







