Translate this page into:
Clinical and Laboratory Markers of Brain Abscess in Tetralogy of Fallot (‘BA-TOF’ Score): Results of a Case–Control Study and Implications for Community Surveillance
Sumit Thakar, MCh Department of Neurological Sciences, Sri Sathya Sai Institute of Higher Medical Sciences Bengaluru 560066 India sumit.thakar@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Cardiogenic brain abscess (CBA) is the commonest noncardiac cause of morbidity and mortality in cyanotic heart disease (CHD). The clinical diagnosis of a CBA is often delayed due to its nonspecific presentations and the scarce availability of computed tomography (CT) imaging in resource-restricted settings. We attempted to identify parameters that reliably point to the diagnosis of a CBA in patients with Tetralogy of Fallot (TOF).
Methods From among 150 children with TOF treated at a tertiary care institute over a 15-year period from 2001 to 2016, 30 consecutive patients with CBAs and 85 age- and sex-matched controls without CBAs were included in this retrospective case–control study. Demographic and clinical features, laboratory investigations, and baseline echocardiographic findings were analyzed for possible correlations with the presence of a CBA.
Statistical Analysis Variables demonstrating significant bivariate correlations with the presence of a CBA were further analyzed using multivariate logistic regression (LR) analysis. Various LR models were tested for their predictive value, and the best model was then validated on a hold-out dataset of 25 patients.
Results Among the 26 variables tested for bivariate associations with the presence of a CBA, some of the clinical, echocardiographic, and laboratory variables demonstrated significant correlations (p < 0.05). LR analysis revealed elevated neutrophil–lymphocyte ratio and erythrocyte sedimentation rate values and a lower age-adjusted resting heart rate percentile to be the strongest independent biomarkers of a CBA. The LR model was statistically significant, (χ2 = 23.72, p = <0.001), and it fitted the data well. It explained 53% (Nagelkerke R 2) of the variance in occurrence of a CBA, and correctly classified 83.93% of cases. The model demonstrated a good predictive value (area under the curve: 0.80) on validation analysis.
Conclusions This study has identified simple clinical and laboratory parameters that can serve as reliable pointers of a CBA in patients with TOF. A scoring model—the ‘BA-TOF’ score—that predicts the occurrence of a CBA has been proposed. Patients with higher scores on the proposed model should be referred urgently for a CT confirmation of the diagnosis. Usage of such a diagnostic aid in resource-limited settings can optimize the pickup rates of a CBA and potentially improve outcomes.
Keywords
markers
cardiogenic brain abscess
Tetralogy of Fallot
Introduction
Cyanotic congenital heart disease (CCHD) accounts for up to 25% of congenital heart disease (CHD) and 12.8 to 69.4% of all brain abscesses, with Tetralogy of Fallot (TOF) being the commonest anomaly associated with a cardiogenic brain abscess (CBA).1 2 3 The hallmark features of TOF are pulmonary valve stenosis, ventricular septal defect (VSD), overriding of aorta, and right ventricular (RV) hypertrophy.4 TOF patients are at substantial risk of developing a CBA, and this risk is further amplified in developing countries where a large percentage of CCHD patients either remain untreated or undergo palliative treatment.5 The clinical presentation of a CBA is often nonspecific, causing delayed diagnosis and treatment. Identification of parameters that point to the diagnosis of a CBA could prove to be a valuable diagnostic aid and potentially improve neurological outcomes of this subset of TOF patients.
Materials and Methods
This was retrospective matched case–control study spanning a 15-year period from 2001 to 2016. Thirty patients with CBAs and 85 age- and sex-matched controls without CBAs were selected from among 150 children with TOF admitted to a tertiary care center. The study was approved by the institutional review board and ethics committee, and has been reported as per the ’Strengthening the Reporting of Observational studies in Epidemiology’ (STROBE) guidelines. All the patients were either untreated or had undergone palliative treatment for TOF prior to their admission in our institute. The diagnosis of a CBA was made based on a contrast-enhanced computed tomography (CT) scan. All patients underwent surgical aspiration either primarily or after a trial of conservative management with empirical antibiotics. Following surgery, all patients received a course of parenteral antibiotics for at least 4 weeks or longer depending on the radiological response. Patients with recurrent CBAs and those with concurrent comorbidities or extracranial infections were excluded from the study.
Selection of Variables
Various possible biomarkers of CBA were recorded from the data stored in the patient charts and the hospital information system. Assuming that severe uncorrected TOF would have a higher risk of harboring a CBA, we included numerous variables that reflected TOF severity. We also selected markers of possible derangements such as coagulopathy,6 infection, or inflammation.7 8 9 10 11 A total of 26 variables including clinical factors, laboratory investigations, chest radiograph findings, and TOF-specific echocardiographic variables were recorded. Clinical data included body mass index, body surface area (BSA), presence of cyanosis, cyanotic spells, dyspnea on exertion, age-adjusted resting heart rate (HR) percentile, and oxygen saturation. The laboratory variables included total white cell count, neutrophil–lymphocyte ratio (NLR), platelet count, erythrocyte sedimentation rate (ESR), bilirubin levels, and coagulation profile. Chest radiographs were screened for the presence of pulmonary oligemia. Baseline echocardiographic variables included the pulmonary artery (PA) index (Nakata index, defined as the sum of the cross-sectional area of the right and left PAs divided by BSA), level of pulmonary stenosis (valvular/ infundibular or both), presence or absence of PA branch confluence, gradient across pulmonary valve, and the type of VSD.
Statistical Analysis
A sample size of 115 (30 cases and 85 controls) with a case–control ratio close to 1:3 was found to be adequate for attaining a power of 90%. For the analysis, 90 patients from the entire sample formed the training group, while 25 constituted the validation group. Continuous variables were expressed as means, while frequency distributions were used to describe the categorical variable. Hypothesis testing was done at each stage for removing outliers and assessing normality. Anderson's normality test was used to evaluate the normality of the data. Bivariate analysis was used to identify preoperative screening markers that correlated with the occurrence of a CBA. Logistic regression (LR) analysis was done using a purposeful variable selection process. Variables with a p-value less than 0.05 in the bivariate analysis were then subjected to a multivariate analysis. Various models were tested for their ability to quantify the impact of each parameter on the outcome. Receiver operator characteristic (ROC) analysis was performed to calculate the area under the curve (AUC) and predictive values for individual variables and the model. The model with the best predictive value was then selected. Validation of the model was performed on a hold-out dataset of 25 patients. An α level of 0.05 was set for all tests.
Results
Demographic, Clinicoradiological, and Laboratory Characteristics
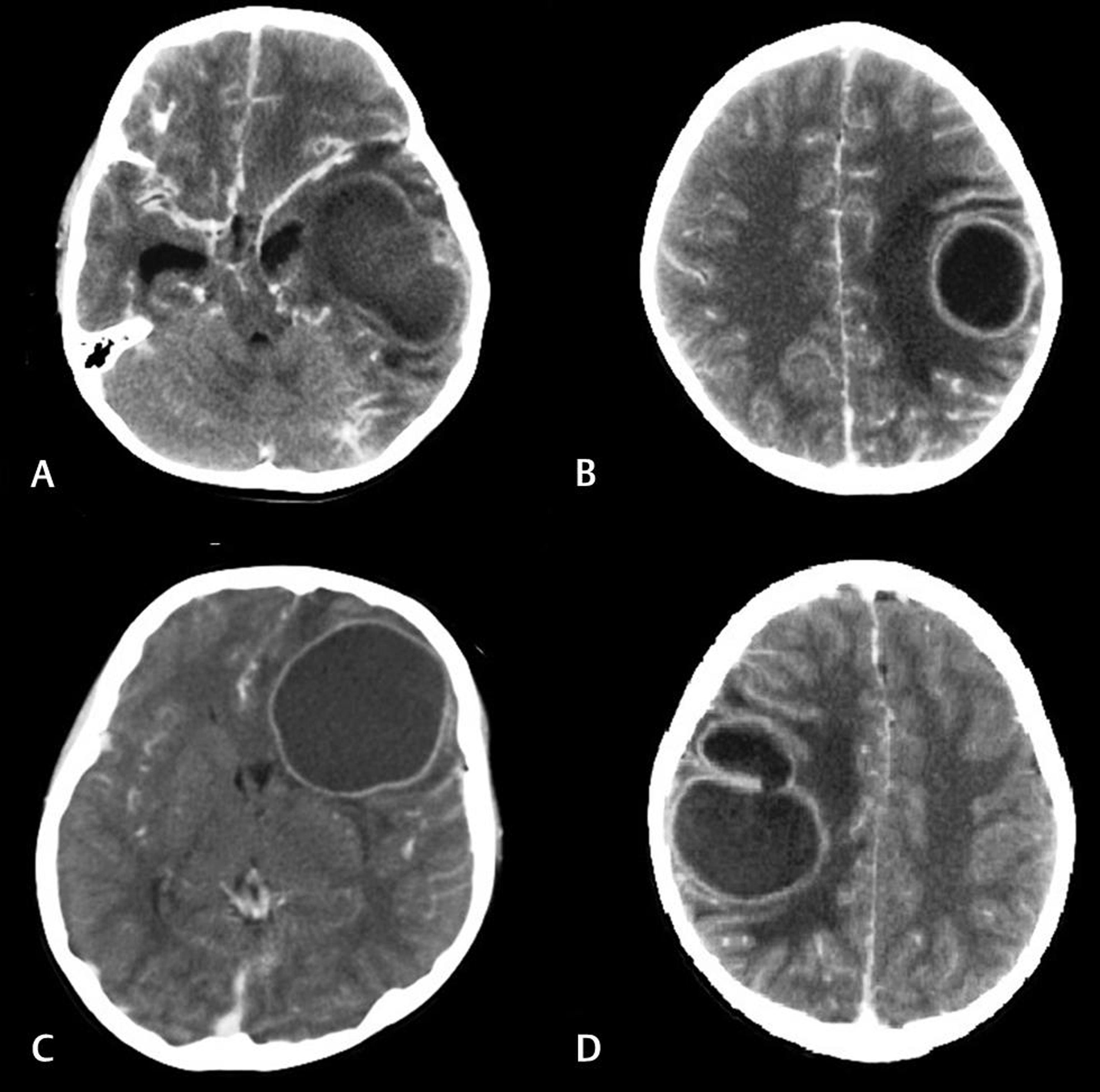
The male: female ratio was 22:8 and the mean age at presentation was 8.54 ± 4.26 years. The common presenting symptoms were headache and fever, while seizures and neurological deficits were seen in a minority of patients. The location of the abscesses (Fig. 1) was as follows: frontal in 12 (40%); parietal in 8 (26.66%); temporal in 5 (16.66%); occipital, basal ganglionic, and thalamic in 1 (3.33%) patient each; and multiple in 2 (6.66%) patients. The pus obtained on surgical aspiration was sterile in all cases.
-
Fig. 1 Contrast-enhanced computed tomography images demonstrating some of the cardiogenic brain abscesses (CBAs) in the study: (A) a left temporal CBA with mass effect on the temporal horn, (B) a left posterior frontal CBA with perilesional edema, (C) a large left frontal CBA with evidence of subfalcine herniation, and (D) a multiloculated abscess in the right posterior frontal region.
Fig. 1 Contrast-enhanced computed tomography images demonstrating some of the cardiogenic brain abscesses (CBAs) in the study: (A) a left temporal CBA with mass effect on the temporal horn, (B) a left posterior frontal CBA with perilesional edema, (C) a large left frontal CBA with evidence of subfalcine herniation, and (D) a multiloculated abscess in the right posterior frontal region.
Bivariate Analysis
Among the 26 variables tested for bivariate associations with the occurrence of CBA, some of the clinical, echocardiographic, and laboratory variables demonstrated significant correlations (p < 0.05) (Table 1). These variables were then subjected to multivariate LR analysis.
|
Clinical variable |
p-Value |
|---|---|
|
Abbreviations: CBA, cardiogenic brain abscess; CXR, chest X-ray; ESR, erythrocyte sedimentation rate; NLR, neutrophil-lymphocyte ratio; PCV, packed cell volume; SpO2, oxygen saturation; VSD, ventricular septal defect. |
|
|
Age |
0.74 |
|
Body mass index |
0.51 |
|
Body surface area |
0.23 |
|
Cyanotic spells |
0.03 |
|
Persistent cyanosis |
<0.001 |
|
Dyspnea on exertion |
<0.001 |
|
Age-adjusted heart rate percentile |
0.001 |
|
SpO2 |
0.83 |
|
Echocardiographic variable |
|
|
Pulmonary artery index |
0.04 |
|
Pulmonary stenosis type |
0.04 |
|
Branch confluence |
0.001 |
|
Pulmonary valve gradient |
0.74 |
|
VSD type |
0.03 |
|
Laboratory variable |
|
|
PCV |
0.65 |
|
Total count |
0.003 |
|
NLR |
<0.001 |
|
Platelet count |
0.30 |
|
ESR |
0.002 |
|
Direct bilirubin |
0.29 |
|
Indirect bilirubin |
0.77 |
|
Total bilirubin |
0.97 |
|
Prothrombin time |
0.19 |
|
Activated partial thromboplastin time |
0.54 |
|
Bleeding time |
0.31 |
|
Clotting time |
0.95 |
|
Radiographic variable |
|
|
Pulmonary oligemia on CXR |
0.26 |
Prediction Model Based on Multivariate LR
The results of the LR analysis using a purposeful variable selection process are shown in Table 2. Among all the variables tested, elevated NLR and ESR values, and a lower age-adjusted resting HR percentile were found to be independent markers of a CBA. The LR model incorporating these variables was statistically significant, χ2 = 23.72, p = <0.001, and it fitted the data well. It explained 53% (Nagelkerke R 2) of the variance in occurrence of a CBA, and correctly classified 83.93% of cases. We tested different models for their ability to predict the outcome. The model with the best prediction was: total points = a + 0.1*b–0.04*c - 1.6, where a = NLR, b = ESR, c = age-adjusted HR percentile and -1.6 is the constant. This model was labelled the ‘BA-TOF’ (Brain abscess in TOF) score.
|
B |
SE |
p-Value |
95% CI (lower) |
95% CI (upper) |
|
|---|---|---|---|---|---|
|
Abbreviations: B, unstandardized coefficient; CBA, cardiogenic brain abscess; CI, confidence interval; ESR, erythrocyte sedimentation rate; NLR, neutrophil-lymphocyte ratio; SE, standard error. |
|||||
|
Constant |
–1.60 |
0.85 |
|||
|
NLR |
0.99 |
0.26 |
<0.001 |
1.63 |
4.45 |
|
ESR |
0.11 |
0.05 |
0.01 |
1.02 |
1.22 |
|
Heart rate percentile |
–0.04 |
0.01 |
0.004 |
0.94 |
0.99 |
ROC Analysis
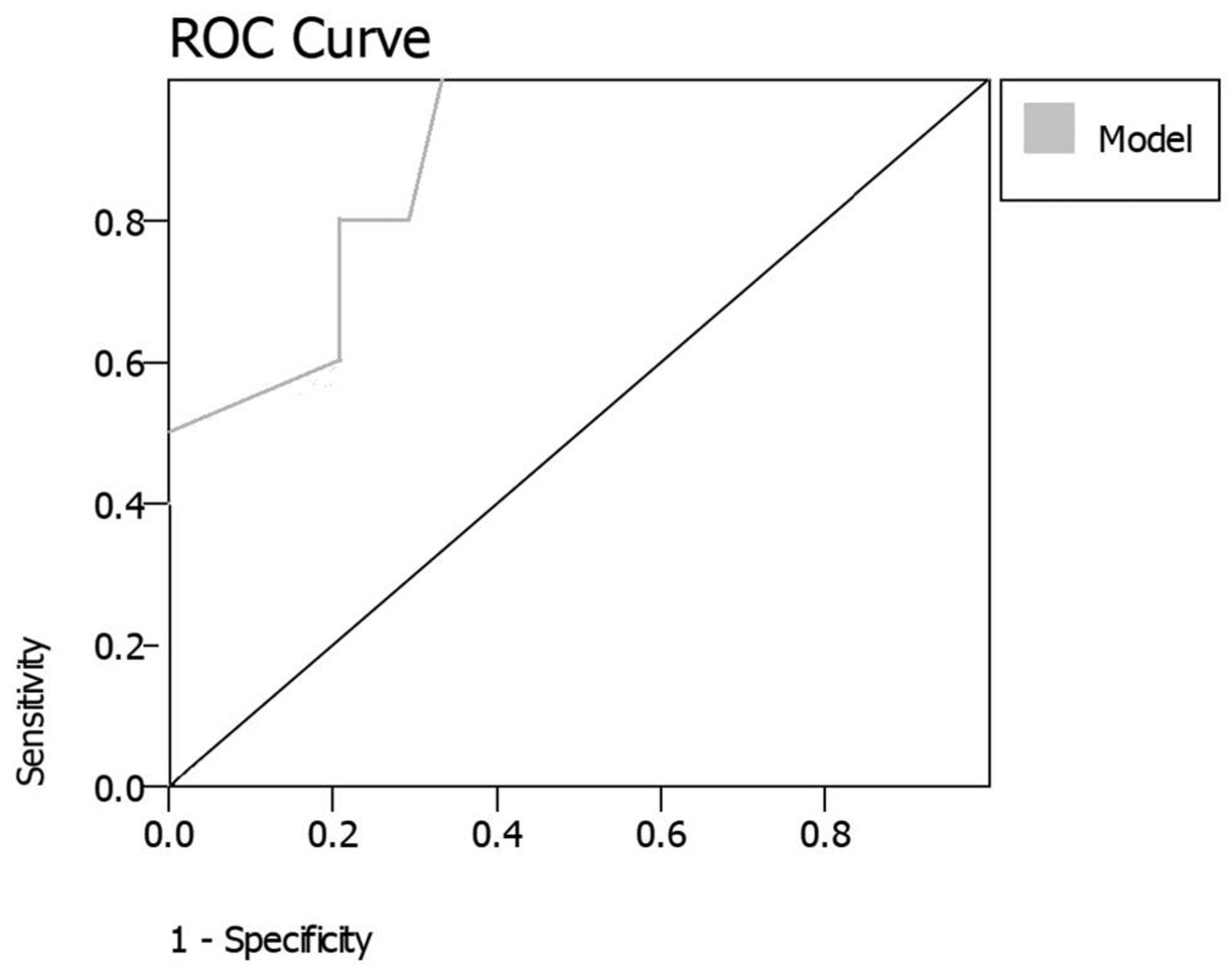
We performed a ROC analysis and calculated the predictive values, thresholds, sensitivity, and specificity of the individual variables and of the LR model score (Table 3). The predictive value of the model (AUC of 0.86) was better than those of the individual variables, and at a threshold of–1, it had a specificity and sensitivity of 80 and 83%, respectively. This indicated that any score >–1 on the model would translate to an 80% chance of the patient harboring a CBA, while a value <–1 on the model would indicate an 83% chance of the patient not having a CBA. For example, a patient with a NLR of 4, an ESR of 30, and HR percentile of 80% would score 2.2 on the model. While this patient would have an 80% chance of harboring a CBA, another patient with a NLR of 1, a mildly elevated ESR of 20 and HR percentile of 40% would score a total of–0.2 on the model, and have an 83% chance of not having a CBA. The model was internally validated on a cohort of 25 patients, and demonstrated good accuracy and predictive value (AUC of 0.80) (Fig. 2).
|
Variable |
AUC |
Threshold |
Sensitivity |
Specificity |
|---|---|---|---|---|
|
Abbreviations: AUC, area under curve; ESR, erythrocyte sedimentation rate; HR, heart rate; NLR, neutrophil-lymphocyte ratio. |
||||
|
NLR |
0.80 |
1.6 |
0.83 |
0.70 |
|
ESR |
0.57 |
15 |
0.25 |
0.90 |
|
HR percentile |
0.31 |
60 |
0.35 |
0.40 |
|
Model |
0.86 |
–1 |
0.83 |
0.80 |
-
Fig. 2 Receiver operator characteristic curve of the regression model when applied on the validation cohort.
Fig. 2 Receiver operator characteristic curve of the regression model when applied on the validation cohort.
Discussion
Global Burden of CBA
The global prevalence of CHD is reported to be 8 to 12/1000 live births.12 13 14 Ninety percent of the affected children are born in nonurban areas with suboptimal access to healthcare.14 CBA is one of the commonest complications and causes for morbidity in patients with CHD. Mortality due to brain abscesses from all causes has significantly decreased over the last few decades after the advent of CT and magnetic resonance imaging,15 16 17 18 both of which, however, still remain elusive in the rural community,19 20 21 where many of the TOF patients are from. This translates to having a potentially large number of untreated TOF patients living with the constant threat of developing a CBA, or risking late detection of a CBA and its associated morbidity and mortality.
CBA-Pathogenesis and Clinical Features
The pathogenesis of a CBA is believed to be multifactorial. Compensatory polycythemia due to decreased oxygen saturation is believed to cause hypoxia and metabolic acidosis.15 22 This leads to the development of ischemic areas within the brain that then become foci for infection. The right-to-left cardiac shunt allows bacteria to bypass the pulmonary circulation and gain access to the cerebral circulation. These microorganisms then eventually seed the ischemic areas, resulting in the formation of an abscess.15 22 Streptococcus, Staphylococcus, and Haemophilus are among the commonly reported organisms.3 15 However, sterile cultures are being increasingly reported due to the usage of broad-spectrum antibiotics.15 In our series, the uniformly negative cultures could be related to the usage of antibiotics—either before admission in some cases or prior to surgical aspiration in patients who were initially managed conservatively. The clinical features of a CBA often pose a diagnostic dilemma. Clinically, the classical triad of fever, headache, and focal neurological deficits is found in less than 30% of the cases.23 24 The commonly encountered symptoms of fever and headache are often nonspecific and are at best poor pointers to the diagnosis of a CBA.23 Confounding the clinical picture further, headaches in TOF patients are known to be related to compensatory cerebral vasodilatation,25 polycythemia-induced hyperviscosity,26 or to migraine in older patients,27 while fever could be related to infective endocarditis or a respiratory tract infection. Symptoms like seizures and focal neurological deficits that would warrant an early CT scan are often seen late in the clinical course, and only in a minority of the patients.23
Utility of Biomarkers
In the light of the ambiguity of the clinical presentation of CBAs, it is often recommended to have a low threshold for performing a CT scan in patients with TOF.15 28 This is, however, not possible in the setting of a predominantly nonurban TOF population alluded to earlier. Usage of reliable nonradiologic biomarkers instead could aid in the diagnosis of a CBA and increase the yield of CT-positive referrals to higher centers. Biomarkers are objective biological characteristics used to measure the presence or progress of disease and the effects of treatment. These include anatomical, laboratory, physiological, radiological, or genetic alterations that are specific to a given disease.29 The utility of these markers in screening, diagnosis, and risk-prediction has been well established in a wide range of diseases.30 31 This study attempted to identify biomarkers of a CBA to enable early pickup of a potentially life-threatening complication of TOF.
Previous Literature and Our Findings
Previous literature with respect to markers or risk factors for CBA is scarce. A study in 1974 had demonstrated a correlation between the severity of the hypoxemia and the development of a CBA in patients with CCHD.32 A similar study published two decades later found that patients with CBAs demonstrated lower base excess and mean oxygen saturation and partial pressure values than controls.3 Both these studies were limited by a suboptimal case:control ratio and the lack of a multivariate analysis. In our bivariate analysis, factors indicative of a more severe TOF (presence of persistent cyanosis, dyspnea on exertion, and the occurrence of cyanotic spells), and a lower HR percentile correlated with the co-existence of a CBA. Among the nonclinical variables, a few echocardiographic and laboratory variables demonstrated significant bivariate correlations with CBA. Multivariate LR demonstrated a combination of elevated NLR and ESR values and a lower HR percentile to be reliable pointers of a CBA.
Implications of Our Findings
NLR, a reflection of the balance between innate and adaptive immune responses, has been found to be raised in several inflammatory conditions.8 In the central nervous system, it has found to be elevated in acute ischemic stroke and, to a lesser extent, in spontaneous intracerebral hemorrhage.33 This is postulated to reflect an imbalanced interaction between the stroke-induced cerebral inflammation and the systemic inflammatory response brought about by mediators that escape from a disrupted blood–brain barrier.33 It is likely that a similar mechanism occurs in a CBA, a condition that has cerebral ischemia as an important part of its pathogenesis. Our finding of an elevated ESR as a biomarker of CBA is backed by previous literature.9 10 11 34 Though often considered nonspecific, inflammatory makers like ESR and C-reactive protein (CRP) have been demonstrated to be elevated in brain abscesses and other intracranial pathologies.9 10 11 34 An “inflammatory index” consisting of these markers was reported to be more prominent in patients with brain abscesses and concomitant meningitis or ventriculitis.9 A study found CRP to be consistently elevated in the early cerebritis phase of cerebral abscesses, and proposed this to as a tool to distinguish them from tumors.10
An interesting finding in our study was the identification of a lower HR percentile as a marker of a CBA. CCHD patients are known to be susceptible to cerebral ischemia due to secondary polycythemia and hyperviscosity.35 It is likely that a lower resting HR would translate to a greater degree of stasis of hyperviscous blood and its related hypoxemic effects. This would in turn facilitate the formation of a CBA.
The ‘BA-TOF’ Score
Our validated, easy-to-use predictive model for the occurrence of a CBA in patients with TOF is based on simple tests that can be performed on the “at-risk” patients with uncorrected or partially corrected TOF at the community level. Though thresholds for the 3 parameters were identified (NLR of 1.6, ESR of 15, and HR percentile of 60%), it should be reiterated that these provide suboptimal results when used in isolation, and that the overall model (‘BA-TOF’ score) provides the highest predictive value. Patients with a total score of >–1 on the model could be referred on priority to higher centers for a CT scan to confirm the diagnosis of a CBA, irrespective of their symptoms. Institution of early treatment would in turn effectively improve clinical outcomes in a population already disadvantaged by high morbidity and mortality rates.36 It may be cautioned that this study has the inherent limitations of a retrospective analysis, and that the model is specific to a subset of the Indian population. This is a preliminary study, and its findings will need prospective validation in larger samples and other populations before it can be widely implemented. It would also be interesting to analyze possible chronological alterations in the identified biomarkers during the natural course and evolution of a CBA.
Conclusions
This study has identified simple clinical and laboratory parameters that can serve as reliable pointers of a CBA in patients with TOF. A scoring model—the ‘BA-TOF’ score—that predicts the occurrence of a CBA has been proposed. Patients with higher scores on the proposed model should be referred urgently for a CT confirmation of the diagnosis. Usage of such a diagnostic aid in resource-restricted settings can optimize the pickup rates of a CBA and potentially improve outcomes.
Ethical Approval
This manuscript has been prepared using guidelines laid down by the institute. The institutional review board and ethics committee have granted approval for the study. The authors confirm that they have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest
None declared.
Authors’ Contributions
V.K. collected data and prepared the initial draft. S.T. was involved in conception, statistical analyses, review, and final preparation of the manuscript. S.A. provided critical feedback and reviewed the manuscript. P.K. reviewed the final manuscript. D.M. supervised the study and reviewed the manuscript draft. A.S.H. provided administrative support and reviewed the final manuscript.
Funding None .
References
- Brain abscess in cyanotic congenital heart disease. Indian Heart J. 1989;41(3):190-193.
- [Google Scholar]
- Brain abscess and congenital heart disease. J Indian Med Assoc. 1990;88(11):312-314. 311
- [Google Scholar]
- Risk factors for brain abscess in patients with congenital cyanotic heart disease. Neurol Med Chir (Tokyo). 1992;32(9):667-670.
- [Google Scholar]
- Natural history of tetralogy of Fallot in infancy. Clinical classification and therapeutic implications. Circulation. 1973;48(2):392-397.
- [Google Scholar]
- Congenital heart disease in India: a status report. Indian Pediatr. 2018;55(12):1075-1082.
- [Google Scholar]
- Coagulopathies in cyanotic cardiac patients: an analysis with three point - of - care testing devices (thromboelastography, rotational thromboelastometry, and Sonoclot analyzer) Ann Card Anaesth. 2017;20(2):212-218.
- [Google Scholar]
- Bilirubin acts as an endogenous regulator of inflammation by disrupting adhesion molecule-mediated leukocyte migration. Inflamm Cell Signal. 2016;3(1):e1178.
- [Google Scholar]
- Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5(1):2.
- [Google Scholar]
- Inflammatory index and treatment of brain abscess. Nagoya J Med Sci. 2012;74:313-324. (3-4)
- [Google Scholar]
- C-reactive protein levels in the differential diagnosis of brain abscesses. J Neurosurg. 1987;67(3):358-360.
- [Google Scholar]
- Erythrocyte sedimentation rate in patients with intracranial mass lesions. Neurology. 1957;7(7):480-482.
- [Google Scholar]
- The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26-34.
- [Google Scholar]
- The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24(4):141-145.
- [Google Scholar]
- The improvement of care for paediatric and congenital cardiac disease across the world: a challenge for the World Society for Pediatric and Congenital Heart Surgery. Cardiol Young. 2008;18(02):63-69.
- [Google Scholar]
- Management of brain abscess in children: review of 130 cases over a period of 21 years. Childs Nerv Syst. 1992;8(7):411-416.
- [Google Scholar]
- Brain abscess in congenital cyanotic heart disease. J Neurosurg. 1983;58(6):913-917.
- [Google Scholar]
- Brain abscess and congenital heart disease. Acta Neurochir (Wien). 1976;33:233-239. (3-4)
- [Google Scholar]
- Lack of CT scanner in a rural emergency department increases inter-facility transfers: a pilot study. BMC Res Notes. 2017;10(1):772.
- [Google Scholar]
- The impact of hospital characteristics on the availability of radiology services at critical access hospitals. J Am Coll Radiol. 2015;12:1351-1356. (12 Pt B)
- [Google Scholar]
- The impact of a rural scanner in overcoming urban versus rural disparities in the utilisation of computed tomography. Aust J Rural Health. 2015;23(3):150-154.
- [Google Scholar]
- Forgotten? Not yet. Cardiogenic brain abscess in children: a case series-based review. World Neurosurg. 2017;107:124-129.
- [Google Scholar]
- 1996. p. :3285-3298. Diagnosis and management of brain abscess. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw-Hill
- Headache and papilledema in an adult with cyanotic congenital heart disease: the importance of fundoscopic evaluation rather than phlebotomy. Congenit Heart Dis. 2012;7(3):E14-E17.
- [Google Scholar]
- Cyanotic congenital heart disease (CCHD) with symptomatic erythrocytosis. J Gen Intern Med. 2007;22(12):1775-1777.
- [Google Scholar]
- Prevalence of migraine headaches in patients with congenital heart disease. Am J Cardiol. 2008;101(3):396-400.
- [Google Scholar]
- Low threshold for intracranial imaging in fever of unknown origin associated with cyanotic heart disease in the pediatric population. Childs Nerv Syst 2020
- [CrossRef] [Google Scholar]
- 1990. p. :3-15. Overview of biological markers. In: Hulka BS, Griffith JD, Wilcosky TC, eds. Biological Markers in Epidemiology. New York: Oxford University Press
- Molecular epidemiology: recent advances and future directions. Carcinogenesis. 2000;21(3):517-524.
- [Google Scholar]
- Risk factors of brain abscess in patients with congenital heart disease. Am J Cardiol. 1974;34(1):97-102.
- [Google Scholar]
- Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated meta-analysis. Front Neurol. 2019;10:1032.
- [Google Scholar]
- Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. 2017;30(1):129-134.
- [Google Scholar]
- Cerebrovascular events in adult patients with cyanotic congenital heart disease. J Am Coll Cardiol. 1996;28(3):768-772.
- [Google Scholar]
- Perioperative complications and clinical out-comes in patients with congenital cyanotic heart disease undergoing surgery for brain abscess. J Neurosci Rural Pract. 2020;11(3):375-380.
- [Google Scholar]






