Translate this page into:
Ankle dorsiflexion assist using a single sensor-based FES: Results from clinical study on patients with stroke
*Corresponding author: Rajdeep Ojha, Center for Advanced Technology Enabled Rehabilitation (CATER), Department of Physical Medicine and Rehabilitation, Christian Medical College, Vellore, Tamil Nadu, India.rajdeep@cmcvellore.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Ojha R, Ezung C, Chalageri PH, Chandy BR, Isaac J, Marimuthu S, et al. Ankle dorsiflexion assist using a single sensor-based FES: Results from clinical study on patients with stroke. J Neurosci Rural Pract 2023;14:48-54.
Abstract
Objective:
Ankle foot orthosis (AFO) commonly prescribed to manage foot-drop following stroke restricts ankle mobility. Commercially available functional electrical stimulation (FES) is an expensive alternative to achieve desired dorsiflexion during swing phase of the gait cycle. An in-house cost-effective innovative solution was designed and developed to address this problem.The aim of the study was to compare spatiotemporal gait characteristics of patients with foot-drop following stroke using commercially available FES against in-house developed versatile single sensor-based FES.
Material and Methods:
Ten patients with cerebrovascular accident of at least 3 months duration and ambulant with/without AFO were recruited prospectively. They were trained with Device-1 (Commercial Device) and Device-2 (In-house developed, Re-Lift) for 7 h over 3 consecutive days with each device. Outcome measures included timed-up-and-go-test (TUG), six-minute-walk-test (6MWT), ten-meter-walk-test (10MWT), physiological cost index (PCI), instrumented gait analysis derived spatiotemporal parameters, and patient satisfaction feedback questionnaire. We calculated intraclass correlation between devices and median interquartile range. Statistical analysis included Wilcoxon-signed-rank-test and F-test (P < 0.05 was considered statistically significant). Bland Altman and scatter plots were plotted for both devices.
Results:
Intraclass correlation coefficient for 6MWT (0.96), 10MWT (0.97), TUG test (0.99), and PCI (0.88) reflected high agreement between the two devices. Scatter plot and Bland Altman plots for the outcome parameters showed good correlation between two FES devices. Patient satisfaction scores were equal for both Device-1 and Device-2. There was statistically significant change in swing phase ankle dorsiflexion.
Conclusions:
The study demonstrated good correlation between commercial FES and Re-Lift suggestive of the utility of low-cost FES device in clinical setting.
Keywords
Stroke
Foot-drop
Functional electrical stimulation
Gait parameters
INTRODUCTION
Foot-drop following stroke can be corrected by functional electrical stimulation (FES) of common peroneal nerve. Swing phase foot clearance is achieved by delivering adequate current at appropriate time in a gait cycle which reduces the compensatory adaptive mechanisms (e.g., circumduction and hip hiking).[1-4] Unlike ankle foot orthosis (AFO), the FES does not restrict ankle mobility.[5,6]
Commonly, tilt sensors, electromyogram, inertial motion units, or heel switches are used in isolation or in combinations to trigger activation of the stimulator. Single, dual, or multi-channel stimulators can be used to stimulate ankle dorsiflexors.[1,7-12] However, commercially available FES devices are expensive. An in-house cost-effective innovative solution was designed and developed to address this problem. The motivation for this study was from our earlier clinical trial comparing FES with AFO in post-stroke foot-drop.[13,14]
We compared spatiotemporal gait characteristics of patients with foot drop following stroke using commercially available FES with an inbuilt tilt sensor (WalkAide) with an in-house developed versatile single sensor-based FES (Re-Lift). Here onward, WalkAide will be called as Device-1 and Re-Lift as Device-2.
MATERIALS AND METHODS
This prospective and clinical trial was conducted after obtaining clearance from the Institutional Review Board and Ethics Committee (CMC/IRB/11978 dated April 2, 2019). Adults with hemiplegia following cerebrovascular accidents, undergoing rehabilitation in the Department of Physical Medicine and Rehabilitation, Christian Medical College, Vellore, were screened for inclusion and exclusion criteria during the study period from July 2019 to December 2020. The key inclusion criteria were ability to walk 20-m with minimal support, ankle dorsiflexor range to at least neutral and being clinically stable, dorsiflexor power less than three (MRC scale), adequate cognition, and communication ability. We excluded patients with conditions such as seizure disorder, deep vein thrombosis of lower limbs, lower extremity ulcers, pacemaker, lower motor neuron lesions affecting common peroneal nerve, pregnancy, plantar flexion contracture, and severe hemi-neglect.
Subjects satisfying the above criteria were recruited after obtaining an informed consent. Participants’ demographic details, stroke localization data, functionality using Modified Barthel Index (MBI), cognition using Addenbrooke Cognitive Examination - 3 (ACE-3), and spasticity of the affected lower limb using Modified Ashworth Scale (MAS) were recorded. All patients underwent uniform comprehensive multi-disciplinary rehabilitation which included gait training on different surfaces, ramps, stair management, and sit to stand balance training. During the standard of care rehabilitation, the recruited subjects received total 7 h of gait training (over 3 days) initially with Device-1 and subsequently another 7 h (over 3 days) with Device-2. The Device-1 [Figure 1] with inbuilt sensor was placed below the level of knee and was calibrated for each individual as per manufacturers’ guidelines. In case of Device-2 [Figure 2], the versatility of sensor permitted its placement either at the level of knee or ankle depending upon the response. In eight subjects, it was placed at the level of knee and in two subjects with stiff knee, it was placed at the dorsum of midfoot. Baseline demographic details were recorded on day-0. The Device-2 had self-calibrating mechanism and did not require any additional calibration for each individual.
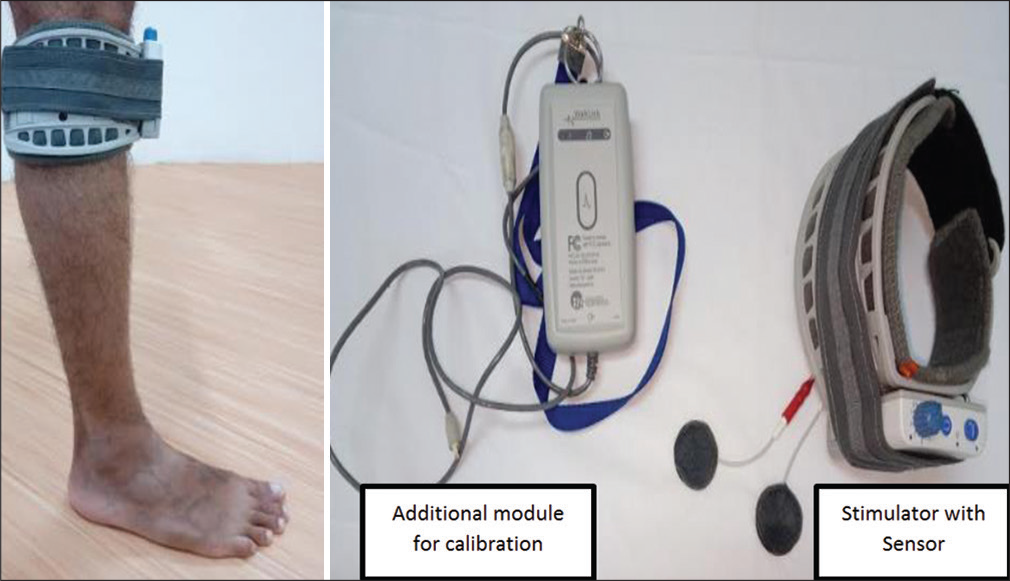
- Commercial FES (Device-1).
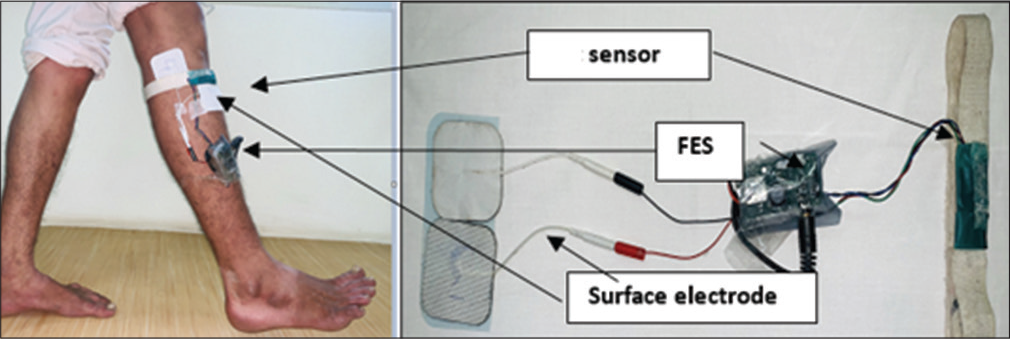
- In-house developed FES, that is, Re-Lift (Device-2).
The primary outcome parameters included Timed up and go test (TUG), Six-minutes-walk-test (6MWT), Ten-meter-walk-test (10MWT), and Physiological cost index (PCI). Calibrated stop watch was used to record 10MWT, 6MWT, and TUG test. Movement Analysis Laboratory at Center for Advance Technology Enabled Rehabilitation (CATER), Department of Physical Medicine and Rehabilitation was used for 3D Instrumented Gait Analysis to derive PCI and secondary parameters such as stance swing ratio, percentage of single limb support, stride length, step-length, and step-width. The data were acquired and analyzed using custom written software.
Median interquartile range (IQR) was calculated for ankle range of motion between pre-swing plantar flexion and maximum swing phase dorsiflexion with Device-1, Device-2, and barefoot. Patient satisfaction score and its analysis was done on the data obtained from the feedback questionnaire as reported in our previous study.[14]
Primary and secondary outcome measures were collected on Day-0 (barefoot), Day-3 (Device-1), and Day-6 (Device-2). Data were screened for outliers and extreme values in Microsoft Excel. Statistical analysis comparing gait parameters, performance, and benefits was done in SPSS v21 and Matlab v2020b. Comparison was done in terms of their performance, gait parameters, benefits, and affordability. The median IQR for each primary and secondary outcome parameters was calculated. The comparison between barefoot versus Device-1 and barefoot versus Device-2 was done using F-test as test of significance. The Wilcoxon-signed-rank test as a non-parametric test was used to compare the outcome measures between Device-1 and Device-2. Statistical significance was considered when P = 0.05.
The outcome parameters using Device-1 and Device-2 were compared using the Bland-Altman plot.[15] The data were analyzed by biostatisticians who were blinded.
RESULTS
Ten patients (nine males and one female) with spastic foot-drop following stroke participated in the study. The mean age, height, and weight were 48.4 ± 9.5 years, 165 ± 6.8 cm, and 66.6 ± 9.4 kg, respectively. Post-stroke duration was <6 months in two subjects, 6–12 months in five subjects, and >12 months three subjects. Among the participants, six had right-sided and four had left-sided hemiplegia. Infarction was the cause of stroke in six subjects (five in middle cerebral artery territory and one in internal capsule region) while four subjects had hemorrhagic stroke in capsuloganglionic region.
Median MBI scores of the study population were 91.3. The study population had ACE-3 score values with a median of 73. The median MAS in hamstring and gastrocnemius muscle on the affected lower limb was 2.
Table 1 shows recorded primary parameters of the ten participants during barefoot, Device-1 and Device-2 trial. The median (IQR) for commercial FES and in-house FES is comparable showing close correlation in outcome measures between the two devices. Further, intraclass correlation coefficient for 6MWT (0.96), 10MWT (0.97), TUG test (0.99), and PCI (0.88) was found to be closer to one reflecting the high agreement between the two devices. The P-value for each of these parameters when compared between Device 1 and Device 2 showed statistically significant intraclass correlation between the two devices. However, on comparison with individual device against barefoot walking (i.e., barefoot vs. Device-1 or barefoot vs. Device-2) outcome parameters did not show statistical significant results despite a trend of improvement for most of the individuals.
| 6MWT (m) | 10MWT (sec) | TUG test (sec) | PCI (beats/meter) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barefoot | Device-1 | Device-2 | Barefoot | Device-1 | Device-2 | Barefoot | Device-1 | Device-2 | Barefoot | Device-1 | Device-2 | |
| P1 | 88 | 113 | 108 | 40 | 25 | 37 | 40 | 34 | 39 | 2.134 | 0.531 | 0.369 |
| P2 | 87 | 92 | 165 | 38 | 37 | 19 | 40 | 40 | 30 | 1.189 | 1.407 | 2.257 |
| P3 | 54 | 72 | 70 | 42 | 50 | 41 | 43 | 54 | 50 | 0.778 | 0.531 | 0.369 |
| P4 | 217 | 225 | 202 | 16 | 28 | 14 | 24 | 20 | 15 | 0.778 | 0.531 | 0.369 |
| P5 | 48 | 34 | 30 | 71 | 110 | 119 | 67 | 120 | 119 | 8.664 | 4.026 | 5.488 |
| P6 | 76 | 56 | 64 | 59 | 46 | 50 | 60 | 52 | 54 | 1.767 | 2.425 | 3.527 |
| P7 | 64 | 62 | 62 | 57 | 42 | 47 | 63 | 50 | 51 | 0.995 | 2.954 | 4.012 |
| P8 | 218 | 255 | 250 | 20 | 20 | 14 | 21 | 20 | 13 | 0.77 | 0.499 | 0.499 |
| P9 | 108 | 122 | 114 | 30 | 23 | 26 | 38 | 24 | 30 | 2.049 | 1.286 | 1.332 |
| P10 | 38 | 41 | 47 | 98 | 82 | 80 | 110 | 94 | 12 | 12.102 | 7.231 | 12.676 |
| Median (IQR) | 81.5 (54, 108) | 82 (56, 122) | 89 (62, 165) | 41 (30, 59) | 39.5 (25, 50) | 39 (19, 50) | 41.5 (38, 63) | 45 (24, 54) | 44.5 (30, 54) | 1.5 (1, 3.75) | 1.00 (1.00, 3.25) | 1.5 (0.0, 4.25) |
| P-value (Barefoot vs. Device) | 0.221 | 0.285 | - | 0.515 | 0.074 | - | 0.314 | 0.139 | 0.167 | 0.676 | ||
| Intraclass correlation | 0.96 | 0.97 | 0.99 | - | 0.88 | |||||||
| Coefficient | (0.86, 0.99) | (0.89, 0.99) | (0.97, 0.99) | (0.52, 0.97) | ||||||||
| P-valve (Device-1 vs. Device-2) | <0.001 | - | <0.001 | - | <0.001 | - | 0.002 | |||||
6MWT: Six-minutes-walk-test, 10MWT: Ten-metres-walk-test, TUG: Timed-up-and-go, PCI: Physiological cost index
Similar analysis on secondary outcome parameters [Table 2] on the paretic side (stride length, single limb support, and walking speed) showed statistically significant intraclass correlation for all the parameters for Device-1 versus Device-2. However, the P-value between barefoot walking against walking with either of Devices-1 or Device-2 was found to be not significant with trend of improvement for most of the subjects. The median, IQR, and intraclass correlation for these parameters were found to be closely related reflecting that both the devices had similar outcomes.
| Stride Length (cm) | Single Limb Support (%) | Walking Speed (cm/s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Barefoot | Device-1 | Device-2 | Barefoot | Device-1 | Device-2 | Barefoot | Device-1 | Device-2 | |
| P1 | 59 | 41 | 54 | 17 | 8 | 12 | 10 | 16 | 10 |
| P2 | 55 | 55 | 57 | 19 | 23 | 25 | 9 | 13 | 12 |
| P3 | 37 | 45 | 37 | 18 | 19 | 22 | 6 | 8 | 8 |
| P4 | 73 | 78 | 88 | 22 | 26 | 23 | 23 | 33 | 34 |
| P5 | 38 | 33 | 33 | 8 | 5 | 6 | 5 | 3 | 2 |
| P6 | 52 | 41 | 50 | 15 | 12 | 15 | 10 | 6 | 9 |
| P7 | 35 | 47 | 54 | 13 | 11 | 13 | 7 | 8 | 10 |
| P8 | 83 | 93 | 93 | 30 | 34 | 33 | 25 | 37 | 39 |
| P9 | 65 | 88 | 73 | 17 | 21 | 25 | 14 | 21 | 18 |
| P10 | 26 | 32 | 37 | 2 | 8 | 5 | 12 | 7 | 6 |
| Median (IQR) | 53.5 (36.5, 67) | 46 (39, 80.5) | 54 (37, 76.75) | 17 (11.75, 19.75) | 15.5 (8, 23.75) | 18.5 (10.5, 25) | 10 (6.75, 16.25) | 10.5 (6.75, 24) | 10 (7.5, 22) |
| P-value (Barefoot vs. Device 1 or 2) | 0.407 | 0.097 | - | 0.383 | 0.161 | - | 0.139 | 0.212 | |
| Intraclass correlation Coefficient | 0.96 (0.86, 0.99) | 0.97 (0.89, 0.99) | 0.99 (0.97, 0.99) | ||||||
| P-value (Device-1 vs. Device-2) | <0.001 | - | <0.001 | - | <0.001 | ||||
Table 3 shows median (IQR) for ankle range of motion (plantarflexion to dorsiflexion) during swing phase in barefoot, Device-1 and Device-2 with P-value 0.024 and 0.014, respectively, demonstrating that FES intervention had significant change in swing phase range of motion.
| Particulars | Barefoot | Device-1 | Device-2 | ||
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | P-value | Median (IQR) | P-value | |
| Swing Phase | 11 (7, 13) | 14.5 (13, 19) | 0.024 | 14.5 (10, 19) | 0.014 |
The scatter plot and the Bland Altman plots for the gait parameters using Device-1 and Device-2 indicate that there is a good correlation between commercial FES and the Re-Lift. Furthermore, most of the points in the Bland Altman plot fall inside the confidence limits suggestive of a good intraclass correlation between two methods for all the parameters.
A sample Bland Altman and Scatter plot for 6MWT is shown in [Figure 3].
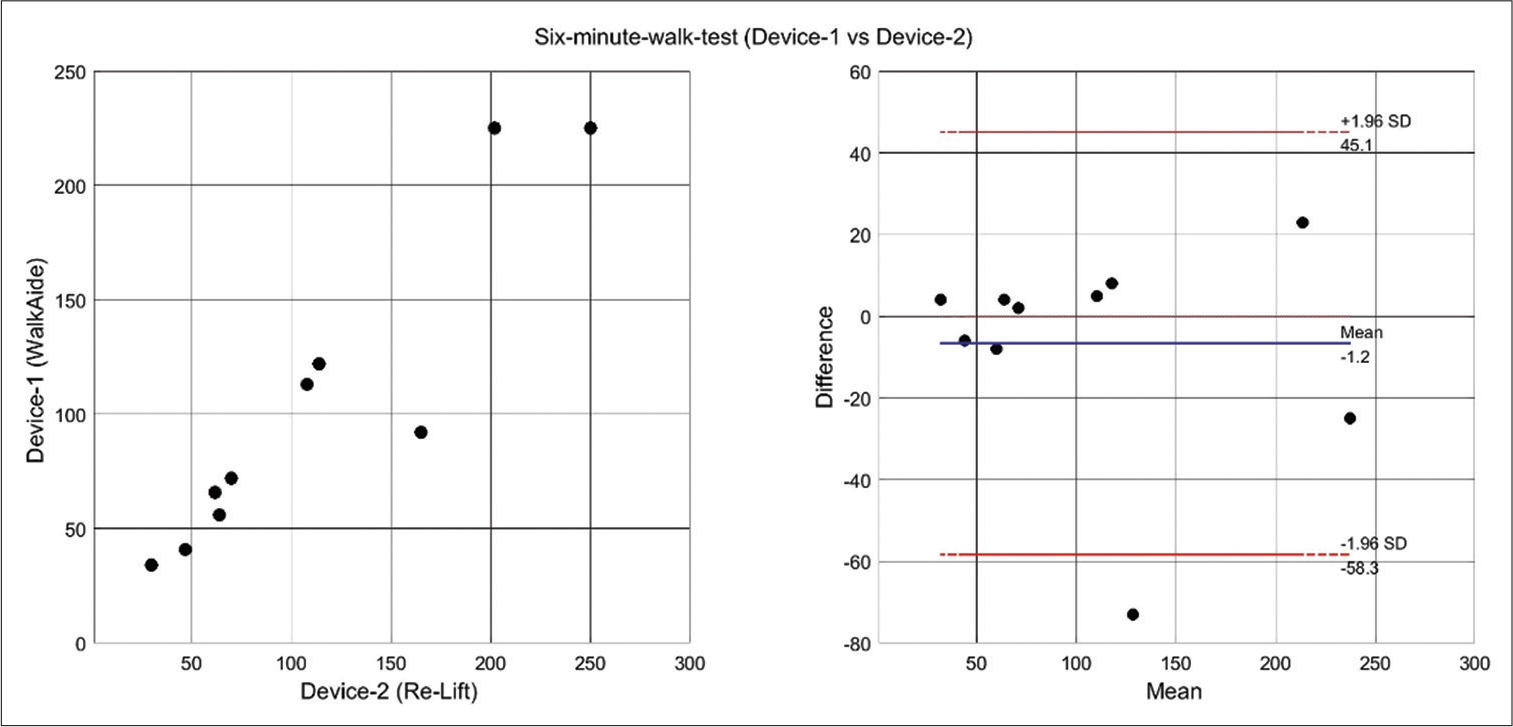
- Scatter plot graph and Bland Altman for Device-1 and Device-2.
Ankle kinematics for a gait cycle of a subject with stiff knee is shown in [Figure 4] (barefoot, AFO, Device-1, and Device-2). The required swing phase dorsiflexion was achieved with device-2 and not with the device-1 because of the flexibility of placement of sensor at the dorsum of mid-foot.
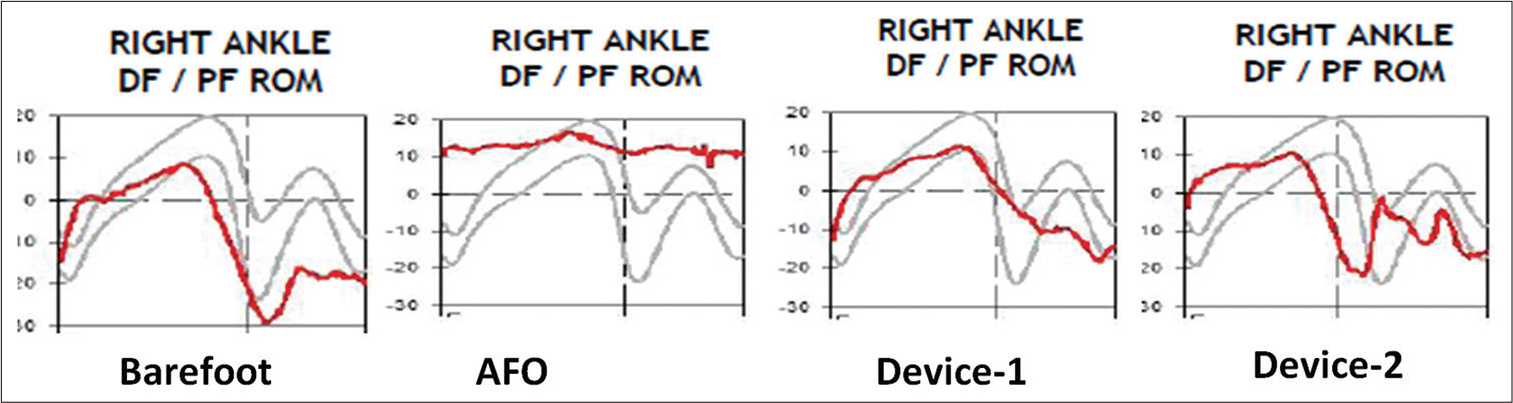
- Comparative sample ankle kinematics for one gait cycle in a subject with stiff knee.
Participants found that both the Device-1 and Device-2 were equally efficient in terms of walking and gave overall rank. However, majority of the participants reported that the Device-1 was better in cosmetic appearance and was easier in donning and doffing.
DISCUSSION
There is an increased in global prevalence of stroke in younger population.[16] Residual paralysis in spastic foot-drop after stroke affects the gait pattern. The conventional treatment with AFO has several setbacks. They restrict ankle mobility, are bulky, and are not cosmetic which leads to poor compliance and rejection.[17]
Significant improvement in gait following foot-drop has been achieved by FES devices. High cost of the commercial FES (cost ranging from INR 2 to 4 lakhs) limits its prescription for everyone. An in-house cost-effective FES Re-Lift was developed from the off-the-shelf components to achieve swing phase dorsiflexion an economical price (costing less than INR 2000 [Bill of Materials]). These two FES modalities were compared in this prospective study.
The mean values of the walking endurance (6MWT) improved with Device-1 (107.2 m) and Device-2 (111.2 m) in comparison to barefoot (99.8 m). Tang et al. have reported that minimally clinically important difference (MCID) for 6MWT in stroke patients is reported as 34.4 m.[18] Better walking endurance was observed in our study; however, it did not meet the MCID criteria of 34.4 m.[19]
The time taken to walk 10 m (i.e., 10MWT) using Device-1 (46. 3 s) and Device-2 (44.7s) was comparable to the baseline, that is, barefoot (46.6 s). Our subjects had a mean walking speed of 0.44 m/s at baseline. In our previous study, it was 0.36 m/s. van Swigchem et al. in their study on post-stroke subjects with high baseline walking speed of 1.02 m/s found no statistical significant change in walking speed with FES. The reported high walking speed could have had a ceiling effect.[14,20]
Patients’ balance and speed can be measured by TUG test.[21] According to Robertson et al., the patients who used FES demonstrated increased balance during walking.[22] The application of FES on dorsiflexor improves the muscle strength thereby reduces the risk of falls. Although the IQR between the Device-1 and Device-2 was in agreement with statistical significance (P < 0.001), clinically mean TUG values were lesser or barefoot (50.8 sec) as compared to FES (Device-1 = 52.3 s and Device-2 =57.3 s). This could be because of shorter period of training.
Physiological cost index assesses patient’s energy expenditure during physical activity. It is a surrogate measure of oxygen consumption during walking.[23] In our study, subjects had less energy consumption with interventions as compared to baseline with mean PCI values of 3.12 (barefoot), 2.62 (Device-1), and 3.08 (Device-2), respectively. This explains the slow speed of barefoot walking.
Secondary outcomes were obtained from instrumented gait analysis. The stride length, single limb support, walking speed, and stance swing ratio were used for analysis. These parameters were assessed to compare the efficiency of the Device-1 and Device-2. The intraclass correlation of the parameters was closer to 1 with P < 0.001 suggesting strong correlation between the devices. However, in comparison to the barefoot walking, the outcome parameters were not statistically significant despite the trend in improvement. This could be attributed to the short duration of training and small sample size.
The change from plantar flexion during pre-swing phase to the maximum dorsiflexion achieved during swing phase of the gait cycle obtained from the instrumented gait analysis demonstrated that the FES intervention was effective in correcting foot-drop as compared to the similar change in barefoot walking. The median IQR for both the devices demonstrated statistically significant ankle dorsiflexion during swing phase of the gait cycle.
The Device-1, that is, WalkAide FES device (Innovative Neutronics, Austin, Texas, USA) uses inbuilt tilt sensor which is placed at the level of knee for sensing the swing phase of the gait cycle. The Device-2 (Re-Lift) is a versatile low cost FES where the sensor can be placed either at the level of knee or ankle to achieve swing phase detection and electrical stimulation of common peroneal nerve producing swing phase dorsiflexion. The study suggests delivering FES to both ankle plantar flexors and dorsiflexor muscles resulted in an 8.6% increase in average swing knee flexion in comparison to FES of ankle dorsiflexors alone.[1] Feedback questionnaire analysis showed patients’ satisfaction in their walking experience, donning and doffing, climbing stairs, and cosmetic appearance with these appliances.
The participants reported a greater satisfaction with both Device-1 and Device-2 than AFO in terms of all the components mentioned in the questionnaire. However, they expressed difficulty in donning and doffing Device-2 as compared to Device-1. This shows a need for a low cost FES device developed for developing countries and should have ease of donning and doffing.[24]
The small sample size and short training duration of the study were the limitations of the study.
CONCLUSION
This prospective study shows that both the devices are equally effective in correcting the foot-drop during the swing phase of the gait cycle in post-stroke patients. Median (IQR) for ankle range of motion (plantar flexion to dorsiflexion) during swing phase was statistically significant between barefoot walking and Device-1 (P = 0.024) and Device-2 (P = 0.014).
A trend of improvement in walking speed and endurance with both FES devices for foot-drop correction, compared to barefoot walking was seen, but they were not statistically significant. Both Device-1 (commercial FES) and Device-2 (in-house built FES - Re-Lift) have shown trend of improvement in gait parameters compared to barefoot walking.
Equal satisfaction level was expressed for both devices in comparison to AFO by all subjects. However, a greater satisfaction for commercial FES expressed with respect to cosmesis and ease of donning/doffing. Overall, results show a good correlation between commercial FES and Re-Lift. As a low-cost FES device in rehabilitating stroke survivors from economically weaker section of the society, Re-Lift looks like a promising solution.
Acknowledgments
The authors thank Fluid Research Grant of Christian Medical College Vellore, Tamil Nadu-India for funding the study. The authors also thank participants who consented for the study.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Fluid Research Grant, Christian Medical College, Vellore.
References
- Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke. 2009;40:3821-7.
- [CrossRef] [PubMed] [Google Scholar]
- Functional electrotherapy: Stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101-5.
- [Google Scholar]
- Feasibility of functional electrical stimulation-assisted neurorehabilitation following stroke in India: A case series. Case Rep Neurol Med. 2012;2012:830873.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in neuroprosthetic management of foot drop: A review. J Neuroeng Rehabil. 2020;17:46.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: A randomized controlled trial. Neurorehabil Neural Repair. 2014;28:688-97.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: A multicenter randomized controlled trial. Neurorehabil Neural Repair. 2013;27:579-91.
- [CrossRef] [PubMed] [Google Scholar]
- Technical developments of functional electrical stimulation to correct drop foot: Sensing, actuation and control strategies. Clin Biomech (Bristol Avon). 2015;30:101-13.
- [CrossRef] [PubMed] [Google Scholar]
- Application of a programmable dual-channel adaptive electrical stimulation system for the control and analysis of gait. J Rehabil Res Dev. 1992;29:41-53.
- [CrossRef] [PubMed] [Google Scholar]
- Autogenic EMG-controlled functional electrical stimulation for ankle dorsiflexion control. J Neurosci Methods. 2010;193:118-25.
- [CrossRef] [PubMed] [Google Scholar]
- Development of 2-Channel Gait Assist Electrical Stimulator for Hemiplegia 3 Osaka, Japan. In: Proceedings of the 41st SICE Annual Conference In: SICE 2002. 2002. p. :2028-31.
- [Google Scholar]
- Development of a prototype of portable FES rehabilitation system for relearning of gait for hemiplegic subjects. Healthc Technol Lett. 2016;3:284-9.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical trial of a prototype of wireless surface fes rehabilitation system in foot drop correction. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:5461-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Gait with Ankle Foot Orthosis (AFO) and Functional Electrical Stimulation (FES) in patients following Stroke. 2019. Available from: https://www.repository-tnmgrmu.ac.in/10954 [Last accessed on 2021 Apr 18]
- [Google Scholar]
- Gait characteristics following stroke: A prospective crossover study to compare ankle-foot orthosis with functional electrical stimulation. Neurol India. 2022;70:1830-5.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25:141-51.
- [CrossRef] [PubMed] [Google Scholar]
- Strokes in young adults: Epidemiology and prevention. Vasc Health Risk Manag. 2015;11:157-64.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of AFO design on walking after stroke: Impact of ankle plantar flexion contracture. Prosthet Orthot Int. 2010;34:277-92.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between perceived and measured changes in walking after stroke. J Neurol Phys Ther. 2012;36:115-21.
- [CrossRef] [PubMed] [Google Scholar]
- Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys Ther. 2010;90:196-208.
- [CrossRef] [PubMed] [Google Scholar]
- Is transcutaneous peroneal stimulation beneficial to patients with chronic stroke using an ankle-foot orthosis? A within-subjects study of patients' satisfaction, walking speed and physical activity level. J Rehabil Med. 2010;42:117-21.
- [CrossRef] [PubMed] [Google Scholar]
- How to identify potential fallers in a stroke unit: Validity indexes of 4 test methods. J Rehabil Med. 2006;38:186-91.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of functional electrical stimulation on balance function and balance confidence in community-dwelling individuals with stroke. Physiother Can. 2010;62:114-9.
- [CrossRef] [PubMed] [Google Scholar]
- Physiological Cost Index as a proxy measure for the oxygen cost of gait in stroke patients. Neurorehabil Neural Repair. 2007;21:429-34.
- [CrossRef] [PubMed] [Google Scholar]
- Functional electrical stimulation (FES) impacted on important aspects of my life: A qualitative exploration of chronic stroke patients' and carers' perceptions of FES in the management of dropped foot. Physiother Theory Pract. 2012;28:1-9.
- [CrossRef] [PubMed] [Google Scholar]







