Translate this page into:
Association of Mean Platelet Volume with Risk Factors and Functional Outcome in Acute Ischemic Stroke
Lubna Zafar, MD 4/1074, Sir Syed Nagar, Near NT Hall, Aligarh 202002, Uttar Pradesh India lubzafar@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Stroke is the second leading cause of death in the world and a major cause of disability, with ischemic stroke contributing to 87% of all strokes. Platelets are central in the formation of thrombus, and in the process, they enlarge in size, become active, and secrete prothrombotic factors. This is supported by the presence of large platelets in ischemic stroke, where they may be implicated in the pathogenesis of vessel occlusion, leading to stroke. The mean platelet volume (MPV) is an important laboratory marker of platelet function and activation.
Materials and Methods The present study was conducted to assess the role of MPV in the pathogenesis, severity, and outcome of ischemic stroke. It was an observational study in 100 acute ischemic stroke (AIS) patients (excluding cardioembolic stroke) admitted to the Medicine wards, Department of Medicine, Jawaharlal Nehru Medical College, a tertiary care hospital at Aligarh. The MPV was correlated with the conventional risk factors of ischemic stroke and outcome (using modified Rankin scale [mRS]). The study revealed statistically significant correlation between MPV and hypertension, type 2 diabetes mellitus, and carotid intima media thickness (CIMT). Also, the MPV at presentation positively correlated with mRS (correlation coefficient 0.818); thus, high MPV was associated with more severe disability.
Conclusion The MPV at the time of presentation of ischemic stroke may be useful in predicting the severity of stroke and neurological recovery. However, a larger study including diverse population is required to endorse its predictive value in AIS.
Keywords
mean platelet volume (MPV)
acute ischemic stroke
modified Rankin scale (mRS)
Introduction
Stroke is the most common neurological disorder worldwide and a leading cause for death and disability in world population after coronary artery disease.1 Acute ischemic stroke (AIS) contributes to 87% of cases, primary intracerebral hemorrhage (ICH) for 10%, and subarachnoid hemorrhage (SAH) for the remaining 3%.2 Stroke is associated with an increased long-term morbidity in terms of physical, behavioral, and cognitive defects. The important risk factors of stroke are hypertension, diabetes mellitus, dyslipidemia, smoking, and cardiovascular disease.3 Platelets have classically been associated with hemostasis and thrombosis. But over the past years, they have been demonstrated to play an integral part in the pathogenesis of atherosclerosis through inflammation and also in its progression. 4 The function and activity of platelets can be determined by measurement of mean platelet volume (MPV), as activated platelets assume large size. It can be correlated with increased platelet aggregation, thromboxane synthesis, β-thromboglobulin release, procoagulant function, and expression of adhesion molecules.5
In myocardial infarction (MI), platelets contribute to thrombus formation after rupture of atherosclerotic plaque in a coronary artery. Hence, increased MPV, indicating more active platelets are a risk for MI.6 Although there have been large number of studies in literature to demonstrate an association between MI and platelet size, very few studies have explored the association between thrombomegaly and ischemic stroke. Hence, an attempt has been made to study the association between MPV and ischemic stroke.
Materials and Methods
The study was an observational, hospital-based study conducted at the Department of Medicine, J.N.M.C.H, AMU, Aligarh from July 2017 to January 2019. The study had institutional ethics committee permission, and the procedures followed in the study were in accordance with institutional guidelines and a predesigned proforma.
Clinically, stroke was defined as focal neurological deficit of abrupt onset lasting more than 24 hours or leading to death with no evidence of a nonvascular cause.7 We recruited 100 adult patients diagnosed with AIS who presented within 48 hours of onset of symptoms. All the participants or valid surrogate respondent of the patients provided informed and written consent before enrolment in the study.
Exclusion Criteria
-
Cardioembolic stroke.
-
Hemorrhagic stroke.
-
Hereditary disorders of large platelets.
-
Patients having thrombocytopenia.
-
Patients with history of hematological malignancies, procoagulant conditions.
-
Patients who were thrombolysed, patients on medications causing thrombocytopenia: hydroxyurea, antineoplastic agents, inhibitors of the platelet integrin αIIbβ3.
-
Patients with history of CKD, CLD, previous stroke.
-
Patients with autoimmune disorders, systemic malignancy, active infection
-
Patients who are unable to give consent due to aphasia, dementia, or altered sensorium and had no valid surrogate respondent.
-
Patients presenting 48 hours after the onset of neurological symptoms.
At baseline, demographic data (age and sex) and history of conventional cerebrovascular risk factors like systemic hypertension, type 2 diabetes mellitus, dyslipidemia and smoking were obtained. The clinical severity at the time of presentation was assessed using modified Rankin's scale (mRS). The MPV was estimated by automated Nihon Kohden MEK-6420p Coulter machine. All data were analyzed by using SPSS software version 23.
Observation and Results
In the study, the mean age of population was 61.22 years. Male preponderance was observed among study subjects, with males (60%) outnumbering females (40%). The mean age of presentation was higher in males (62.53 ± 10.627 years) as compared with females (59.25 ± 13.61 years). The most common risk factor for stroke was hypertension (64%), followed by type 2 diabetes mellitus (42%) (Table 1).
|
Number |
Percentage (%) |
|
|---|---|---|
|
Male |
60 |
60 |
|
Female |
40 |
40 |
|
< 40 years |
6 |
6 |
|
41–60 years |
46 |
46 |
|
> 60 years |
48 |
48 |
|
Smoker |
40 |
40 |
|
Nonsmoker |
60 |
60 |
|
Alcoholic |
16 |
16 |
|
Nonalcoholic |
84 |
84 |
|
Hypertensive |
64 |
64 |
|
Nonhypertensive |
36 |
36 |
|
Diabetic |
42 |
42 |
|
Nondiabetic |
58 |
58 |
|
LDL-C < 130 mg/dL |
45 |
45 |
|
TG > 150 mg/dL |
69 |
69 |
|
TC > 200 mg/dL |
60 |
60 |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
The pattern of MPV in the patients was observed; 40% of patients had MPV between 5 to 7.5 fl, 30% had MPV between 7.5 to 10 fl, and 27% had MPV above 10 fl.
The mean MPV of hypertensives was 9.570 ± 1.9961 fl and of nonhypertensives was 6.169 ± 0. 9899 fl (p-value – 0.00, p < 0.05). The mean MPV of patients having HbA1C ≥ 6.5 and HbA1C < 6.5 was 10.013 ± 1.7581 fl and 6.466 ± 1.2990 fl, respectively (p-value – 0.045, p < 0.05). The mean MPV of patients with carotid intima media thickness (CIMT) ≥ 0.7 mm was 9.557 ± 2.2095 fl, while mean MPV of subjects with CIMT < 0.7 mm was 6.805 ± 1.4988 fl (p-value – 0.022, p < 0.05). Thus, among the risk factors for ischemic stroke hypertension, type 2 diabetes and CIMT were found to be significantly associated with MPV (Table 2).
|
Risk factors |
n |
Mean MPV |
Standard deviation |
Standard error mean |
p-Value |
|---|---|---|---|---|---|
|
Gender |
|||||
|
Male |
60 |
8.623 |
2.4422 |
0.3153 |
0.302 |
|
Female |
40 |
7.930 |
2.1976 |
0.3475 |
|
|
Age (years) |
|||||
|
< 60 |
36 |
7.992 |
2.3728 |
0.3955 |
0.911 |
|
≥ 60 |
64 |
8.584 |
2.3389 |
0.2924 |
|
|
Smoking |
|||||
|
Smoker |
40 |
9.533 |
2.2156 |
0.3503 |
0.255 |
|
Non-smoker |
60 |
7.555 |
2.1253 |
0.2744 |
|
|
Alcoholism |
|||||
|
Alcoholic |
16 |
10.713 |
1.7689 |
0.4422 |
0.038 |
|
Nonalcoholic |
84 |
7.895 |
2.1903 |
0.2390 |
|
|
LDL-C |
|||||
|
≥ 130 |
45 |
8.547 |
2.3783 |
0.3545 |
0.874 |
|
< 130 |
55 |
8.182 |
2.3555 |
0.3176 |
|
|
Hypertension |
|||||
|
Hypertensive |
64 |
9.570 |
1.9961 |
0.2495 |
0.000 |
|
Nonhypertensive |
36 |
6.169 |
0.9899 |
0.1650 |
|
|
HbA1C |
|||||
|
≥ 6.5 |
53 |
10.013 |
1.7581 |
0.2415 |
0.045 |
|
< 6.5 |
47 |
6.466 |
1.2990 |
0.1895 |
|
|
CIMT (mm) |
|||||
|
≥ 0.7 |
56 |
9.557 |
2.2095 |
0.2953 |
0.022 |
|
< 0.7 |
44 |
6.805 |
1.4988 |
0.2260 |
|
Abbreviations: AIS, acute ischemic stroke; CIMT, carotid intima media thickness; LDL-C, low-density lipoprotein cholesterol; MPV, mean platelet volume.
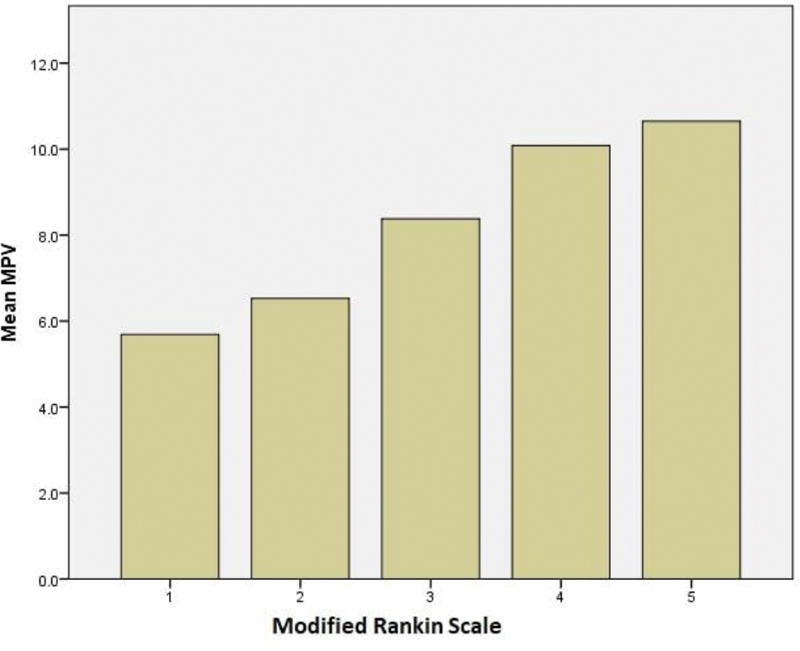
The clinical severity of stroke was assessed using mRS at the time of admission. The scale ranges from 0 to 6 with mRS 0 signifying no disability and a score of 6 implying death. As much as 24% of patients had mRS 2, 23% had mRS 4, 21% had mRS 5, and 17% had mRS 1. There were no patients with mRS 0 and 6. The MPV of patients in each mRS was compared. The mean MPV of study population with mRS 1, 2, and 3 were 5.7 fl, 6.5 fl, and 8.4 fl, respectively, while the patients having severe functional impairment with mRS 4 and mRS 5 were having MPV 10.1 fl and 10.6 fl, respectively (Fig. 1).
-
Fig. 1 Clinical severity of stroke (modified Rankin scale [mRS]) and mean platelet volume (MPV) in study population.
Fig. 1 Clinical severity of stroke (modified Rankin scale [mRS]) and mean platelet volume (MPV) in study population.
The study subjects were categorized into two groups, based on mRS. Group 1 included patients with mRS ≤ 2 and group 2 patients had mRS ≥ 3. The mean MPV of group 2 was 9.851 ± 1.7604 fl, which was significantly higher than mean MPV of group 1 (6.180 ± 1.0743 fl). This result was statistically significant. (p value – 0.001, p < 0.05). The correlation of MPV with mRS was calculated using Pearson formula. Pearson correlation coefficient was 0.818; thus, the correlation was statistically significant (Table 3; Fig. 2).
|
MPV |
mRS |
||
|---|---|---|---|
|
MPV |
Pearson correlation |
1 |
0.818a |
|
Sig. (2-tailed) |
0.000 |
||
|
N |
100 |
100 |
|
|
mRS |
Pearson correlation |
0.818a |
1 |
|
Sig. (2-tailed) |
0.000 |
||
|
N |
100 |
100 |
Abbreviations: MPV, mean platelet volume; mRS, modified Rankin Scale.
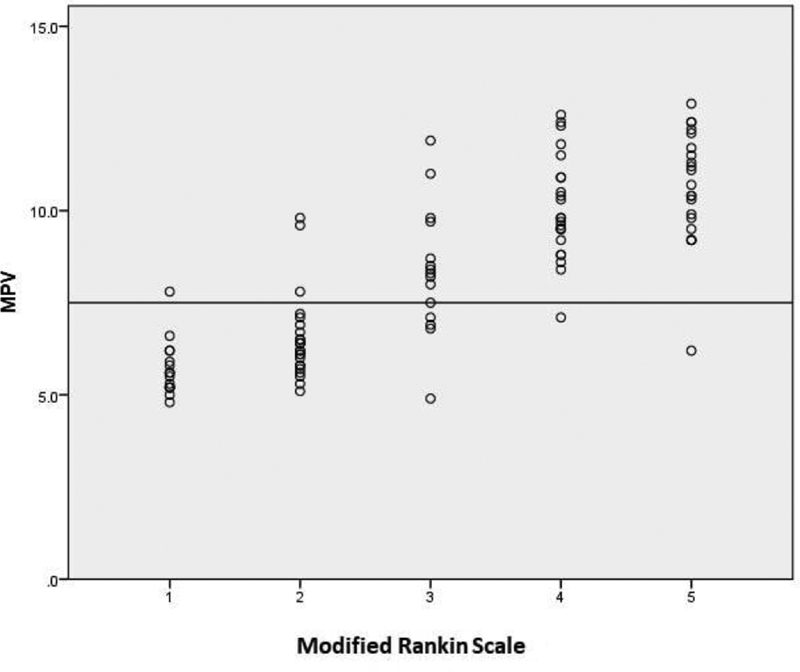
-
Fig. 2 Modified Rankin scale (mRS) and mean platelet volume (MPV) correlation analysis.
Fig. 2 Modified Rankin scale (mRS) and mean platelet volume (MPV) correlation analysis.
Discussion
The mean age of study subjects was 61.22 ± 11.9 years, which is much lower compared with Western studies, as reported by O'Malley et al (79.5 ± 6.5 years),8 Pikija et al (78),9 Muscari et al (78),10 and is comparable to a study done by Bath et al (65 ± 9 years).5 Thus, it signifies that the mean age of Indian population is less compared with Western studies, as reported by Shah et al, where mean age of ischemic stroke was 58 years.11 The mean age of female participants was 59.25 ± 13.61 years, whereas the mean age of males was 62.53 ± 10.63 years. In a recent study among the urban Indian population, the incidence of ischemic stroke was higher in males than in females less than 65 years but dramatically increased after 65 years of age.12 The prevalence of modifiable risk factors like smoking and drinking is more among Indian males than females.13
The major risk factors for stroke were studied; among them, hypertension (64%) was the most prevalent risk factor present in study subjects. The results were comparable to a study among the urban population in Calcutta in 2001, where hypertension was found to be the most common risk factor.14 The studies conducted by Muscari et al (84.7%)10 and Pikija et al (82.7%)9 also showed hypertension as the most prevalent risk factor.
The mean MPV of study patients was 8.392 ± 2.3609 fl. The mean MPV of study subjects by Shah et al11 was 11.86 + 0.96 fl and by O'malley T et al8 was 11. 3 fl. The mean MPV values of study by Butterworth and Bath were 8.04 ± 1.04 (7.69 ± 0.83) for EDTA and 7.35 ± 1.05 (7.09 ± 0.74) for citrate.7 The mean MPV in study by Muscari et al in stroke patients was 8.30 ± 1.10 fl.10 This variation in MPV may be attributed to the type of anticoagulant used, as EDTA leads to more swelling of platelets compared with citrate. Also, the timing of sample collection after stroke and the type of automated cell analyzers used in different studies may contribute to this discrepancy in the values of MPV.
In our study, average MPV in hypertensives was 9.570 fl and in nonhypertensives was 6.169fl. This difference in MPV was statistically significant between the two groups with p-value of 0. 000. Similar observations were made by Coban et al15 and Varol et al,16 who showed significant elevation of MPV in hypertensives. Coban et al also observed positive correlation of MPV with ambulatory diastolic blood pressure in essential hypertension (p < 0.05) and postulated that activated platelets, represented as MPV in laboratory parameters, are actively involved in atherogenesis. Even the prehypertensive patients had statistically significant elevation of MPV compared with controls.16 Gang in a retrospective cohort study observed that elevated MPV was associated with hypertension, independent of other risk factors for hypertension like age, sex, waist circumference, creatinine, and smoking.17
Diabetes being a procoagulant state, patients are at risk of any thrombotic event. In our study, the glycemic control of patients was compared with MPV. The mean MPV of patients having HbA1c more than or equal to 6.5 was 10.013, while mean MPV of those with HbA1c less than 6.4 was 6.466. The p-value was 0.045(p < 0.05); thus, the result was significant. A study by Patil et al showed that MPV in patients with diabetes mellitus was significantly high (10.16 ± 0.89 fL) compared with nondiabetic patients (8.25 ± 0.91 fL; p < 0.001).1 The increase in the platelet activity may be attributed to the osmotic effect of glucose, nonenzymatic glycation of platelet membrane proteins, and activation of protein kinase C.18
The normal range of CIMT in healthy adults is 0.6 to 0.7 mm and values ≥ 1 mm are considered a risk factor for stroke. Jacoby et al19 and Lorenz et al20 observed that CIMT value has a direct correlation with ischemic stroke and may be a noninvasive predictor of future ischemic stroke incidence.
The mean MPV of patients with CIMT 0.7 mm or more was 9.557 fl, while mean MPV was 6.805 fl in patients with CIMT less than 0.7 mm. This difference was statistically significant with p-value of 0.022 (p < 0.05). In a study by Ma et al,21 MPV was found to be independently associated with the CIMT in adult subjects in the absence of conventional stroke risk factors like dyslipidemia, hypertension, and diabetes mellitus. Arévalo-Lorido et al conducted a study to observe MPV and CIMT in atherothrombotic ischemic stroke patients, and they observed that MPV higher than 11.25 fl significantly correlated with severity of carotid stenosis.22
The association of MPV with stroke severity was assessed by comparing the mRS with corresponding mean values of MPV in each group. On the basis of mRS, patients were divided into two groups. Group 1 included patients having mRS 0, 1 and 2, while group 2 had patients with score 3 or more. The mean MPV of group 1 was 6.180 fl, while the mean MPV of group 2 was 9.851 fl. The difference between the two groups had a p-value of 0.001; thus, the result was significant. Therefore, concluding that higher MPV was associated with poor outcome. This is consistent with a study by Ghahremanfard et al23 in which MPV within the first 24 hours of ischemic stroke was correlated with the severity of stroke, assessed by mRS. After adjusting for confounding factors associated with ischemic stroke in multivariate regression model, the association of MPV with stroke severity remained statistically significant (p = 0.012)
In an Indian study by Sarkar et al, the motor deficits in nondiabetic, nonhypertensive ischemic stroke patients was assessed using Canadian Neurological Scale (CNS) and compared with platelet indices. The patients having lower CNS score (more neurological deficit) had higher MPV (r = 0.46, p < 0.05).24
Mohamed et al assessed the association of MPV at presentation and functional outcome, determined by mRS at 3 months in patients of ischemic stroke. The MPV in unfavorable group (mRS ≥ 3) was 10.4 ± 2.3 fL and in the favorable group (mRS 0–2) was 8.7 ± 1.3 fL (p < 0. 001), independent of other risk factors.25
In addition to MPV, Xie evaluated the association of other platelet indices with the neurological outcome in AIS patients who underwent thrombolysis. The outcome of the participants was assessed at 90 days after stroke, using mRS, and values between 3 to 6 were defined as unfavorable outcome. After multivariate logistic regression analysis, it was revealed that MPV and platelet distribution width (PDW) were independently related to poor outcome at 90 days after ischemic stroke.26 A similar study was conducted by Inanc et al in AIS patients receiving intravenous thrombolysis. The neurological impairment of participants was evaluated at 24 hours and 3 months after the onset of stroke, using National Institutes of Health Stroke Scale (NIHSS). MPV was significantly correlated with NIHSS at 24 hours (r = 0.221, p = 0.012) and at 3 months (r-0.249, p = 0.005). They also postulated that increased MPV may be suggestive of increase in reactivity of platelets, which contribute to pathogenesis of AIS.27
The role of inflammation in the destabilization of atherosclerotic plaque is well-established, which results in ischemic vascular events. This inflammatory environment leads to increased expression of P-selectin and E-selectin, contributing to adhesion of platelets, which, in turn, become activated and secrete various inflammatory mediaters.28 IL3 and IL6 may influence megakaryocyte size and produce larger and more activated platelets.29
Conclusion
Our study exhibits significant correlation of MPV with hypertension, type 2 diabetes mellitus, and CIMT in ischemic stroke. It also established a statistically significant correlation between clinical severity of stroke (mRS) and MPV in AIS. Thus, suggesting the role of larger platelets in the pathogenesis of ischemic stroke and possible use of MPV as a prognostic indicator.
Limitations of the Study
The study being a single center study, including limited patients, cannot be generalized to the entire population. The stroke severity was assessed only at presentation and correlated with MPV, but the functional recovery after discharge of the patients was not evaluated.
Thus, there is need for multicenter studies, including diverse populations, to extrapolate these results. Also, in vitro studies are required to explore interventions, in order to regulate thrombomegaly and thus the predisposition to ischemic events.
Conflict of Interest
None declared.
Funding None.
References
- Association of mean platelet volume with acute ischemic cerebrovascular accident among patients with type 2 diabetes mellitus: a hospital-based study. J Assoc Physicians India. 2018;8:44-47.
- [Google Scholar]
- Cerebrovascular diseases. Adam‘s and Victor‘s Principles of Neurology. 9th ed. United States of America: McGraw Hill; 2009. p. :545-650.
- [Google Scholar]
- Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11(2):61-71.
- [Google Scholar]
- Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157-161.
- [Google Scholar]
- Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399-404.
- [Google Scholar]
- The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets. 1998;9:359-364.
- [Google Scholar]
- Higher mean platelet volume determined shortly after the symptom onset in acute ischemic stroke patients is associated with a larger infarct volume on CT brain scans and with worse clinical outcome. Clin Neurol Neurosurg. 2009;111:568-573.
- [Google Scholar]
- Meanplatelet volume (MPV) increase during acute non-lacunar ischemic strokes. Thromb Res. 2008;3:25-30.
- [Google Scholar]
- Incidence of stroke in adults according to age, sex and subtypes in urban Indian population. Neurol Neurosci Rep. 2020;3:1-4.
- [Google Scholar]
- Stroke in urban population of Calcutta- An epidemiological study. Neuroepidemiology. 2001;2(3):201-207.
- [Google Scholar]
- The mean platelet volume in patients with essential and white coat hypertension. Platelets. 2005;16(7):435-438.
- [Google Scholar]
- Mean platelet volume in patients with prehypertension and hypertension. Clin Hemorheol Microcirc. 2010;45(1):67-72.
- [Google Scholar]
- Association between mean platelet volume and hypertension incidence. Hypertens Res. 2017;40:779-784.
- [Google Scholar]
- Study of impact of glycemic status (HbA1c) on platelet activity measured by mean platelet volume & vascular complications in diabetics. J Assoc Physicians India. 2019;67(4):26-29.
- [Google Scholar]
- Noninvasive atherosclerosis imaging for predicting cardiovascular events and assessing therapeutic interventions. Curr Atheroscler Rep. 2004;6:20-26.
- [Google Scholar]
- Prediction of clinical cardiovascular events with carotid intima-media thicknes, a systematic review and meta-analysis. Circulation. 2007;115:459-467.
- [Google Scholar]
- Mean platelet volume in relation to carotid atherosclerosis in normotensive, euglycemic, and normolipidemic Chinese middle-aged and elderly adults. Angiology. 2014;65(6):512-518.
- [Google Scholar]
- Mean platelet volume predicting carotid atherosclerosis in atherothrombotic ischemic stroke. Ir J Med Sci. 2012;181:179-183.
- [Google Scholar]
- The relationship between mean platelet volume and severity of acute ischemic brain stroke. Neurosciences. 2013;18(2):147-151.
- [Google Scholar]
- Platelet indices as a marker of severity in non-diabetic non-hypertensive acute ischemic stroke patients. J Assoc Physicians India. 2018;66:40-42. (July):
- [Google Scholar]
- The mean platelet volume and plateletcrit as predictors of short-term outcome of acute ischemic stroke. Egypt J Neurol Psychiat Neurosurg. 2019;55:4.
- [Google Scholar]
- Platelet volume indices for the prognosis of acute ischemic stroke patients with intravenous thrombolysis. Int J Neurosci. 2019;129(4):344-349.
- [Google Scholar]
- MPV, CRP, and prognosis in AIS treated with IV thrombolytics. Med Sci Monit. 2018;24:3782-3788.
- [Google Scholar]
- The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364(18):1746-1760.
- [Google Scholar]
- Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688-1691.
- [Google Scholar]






