Translate this page into:
Carotid plaque volume measurement in ischemic stroke: A pilot study
*Corresponding author: Kiren George Koshy, Department of Neurology, NIMHANS, Bengaluru, Karnataka, India. kirenkoshy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Koshy KG, Kumar V, Ramakrishnan S, Kenchaiah R, Arvinda HR. Carotid plaque volume measurement in ischemic stroke: A pilot study. J Neurosci Rural Pract. doi: 10.25259/JNRP_578_2023
Abstract
Objectives:
Carotid atherosclerosis is a significant risk factor for acute ischemic stroke. Three-dimensional (3D) sonography is a new technique that can be used to analyze carotid plaque both quantitatively and qualitatively. The aim was to study carotid atherosclerosis in stroke patients and healthy controls in terms of plaque volume quantification by 3D ultrasound (US).
Materials and Methods:
An observational descriptive study was conducted in the stroke unit of a university referral hospital in South India. Patients with ischemic stroke between the ages of 20 and 70 years were studied, along with age- and sex-matched controls. Carotid sonography (2D and 3D) along with Doppler studies was done in all patients, using Philips Affiniti 50 US system. Vascular plaque quantification software was used to assess plaque volume.
Results:
Twenty-four subjects were recruited, and two were excluded from the study. Twelve were cases, and ten were controls. The mean carotid intima-media thickness (CIMT) (average CIMT in six sites in common carotid artery, three on each side) studied in patients was 0.65 ± 0.10 mm, while that for healthy subjects was 0.62 ± 0.06 mm. The mean plaque volume in patients with stroke or transient ischemic attack was 179.82 ± 310.3 mm3, and that in healthy subjects was 56.75 ± 69.6 mm3. Plaque heterogeneity, surface irregularity, and ulceration were found to be common in symptomatic carotid plaques.
Conclusion:
The 3D sonography is a non-invasive and simple feasible tool for the analysis of carotid plaque.
Keywords
Stroke
Carotid plaque
Plaque volume
Ultrasound
INTRODUCTION
The management of ischemic stroke depends on its etiology and mechanism. Vessel imaging (by ultrasound [US], computed tomography angiography, or magnetic resonance angiography) helps determine the mechanism of stroke and in ruling out large-vessel atherosclerosis. This is important, both in acute stroke, to assess the need for mechanical thrombectomy and also in the context of symptomatic or asymptomatic carotid stenosis for deciding on further treatment.
Carotid atheromatous plaques can cause a stroke by large vessel occlusion or by artery-to-artery embolism. The most studied methods for atherosclerosis burden assessment include coronary artery calcium score (CACS) and carotid US for measuring intima-media thickness (IMT) and carotid plaque thickness or volume. Carotid ultrasonography is useful in measuring IMT, detecting plaque, estimating luminal stenosis, and assessing plaque morphology and echogenicity. Echolucent plaques (which are lipid-rich) are associated with an increased risk of stroke.[1] Plaque echogenicity, total area, and surface irregularities are surrogate markers of plaque instability. Studies have shown that total plaque area better estimates the atherosclerotic burden than IMT.[1,2]
According to the 2016 European guidelines, a carotid IMT (CIMT) of 1.0 mm or more is considered to be increased. The Mannheim CIMT Consensus suggests that carotid plaque, stenosis, and occlusion are cardiovascular risk markers. The newly introduced niche technology of three-dimensional (3D) carotid Doppler examination offers an excellent view of the plaque in all three dimensions and better quantification of plaque in terms of plaque volume. Plaque volume can be used to monitor patients on medical management serially and to observe for progression or regression of plaque. The technique also enables better characterization of atheromatous plaques and their qualitative features. The 3D plaque imaging offers a more precise estimation of plaque burden, and serial scanning with plaque volume measurement is useful in assessing the response to treatment. It also serves as an additional marker in asymptomatic carotid stenosis as there is a need to identify risk for future plaque disruption and artery-to-artery embolism.[3]
The initially described methods of 3D sonography were manual freehand techniques, which were technically challenging and time-consuming.[4] Over the past decade, semi-automated single-sweep 3D US imaging has been developed.[5] Kalashyan et al. studied this automated 3D sonography technique in 79 patients with transient ischemic attack (TIA) or stroke and established a good interobserver agreement for plaque volume measurement.[6] A similar study by Johri et al. confirmed the good interobserver agreement in carotid plaque volume measurement in 10 patients with coronary artery disease (CAD).[7] Krasinski et al. used 3D carotid sonography to study the regression of vessel wall volume in patients with asymptomatic carotid stenosis following treatment with statins.[8] Makris et al. evaluated seven studies of plaque volume by 3D sonography.[9] Intraobserver variability in these studies ranged from 2.8% to 6.0%, while interobserver variability ranged from 4.2% to 7.6%. In four studies, 3D plaque volume was superior to CIMT in assessing response to treatment.[10-13] The 5-year risk of stroke, myocardial infarction (MI), and vascular death is higher for plaques with a higher cross-sectional area (CSA). In a study of 6101 asymptomatic patients, by 3D-based US approach, carotid plaque burden was highly correlated with CACS.[14] A recent study has reported that plaque echolucency is correlated with symptomatic status in patients with moderate carotid artery stenosis.[15]
Studies have established a good interobserver agreement for plaque volume measurement by 3D carotid sonography. 3D carotid imaging is also useful in detecting milder degrees of stenosis, floating plaques, and ulcerated plaques, compared to magnetic resonance imaging.[16] However, studies based on the latest automated technique incorporating qualitative parameters are limited due to the cost of the US probe and the need for specialized laboratories. The 3D sonography is not performed in routine clinical practice, and there are no prior studies from India on 3D carotid imaging. Our study aims to fill this gap by studying patients with stroke and healthy subjects by 3D carotid sonography in quantitative and qualitative plaque analysis.
MATERIALS AND METHODS
The study is a prospective descriptive study of consecutive patients with stroke and age- and sex-matched healthy subjects.
Participants
Consenting patients aged between 20 and 70 years with ischemic stroke presenting to the Stroke Unit of a university referral hospital were included in the study. Exclusion criteria included strokes secondary to venous sinus thrombosis and intracerebral hemorrhage. Age- and sex-matched healthy individuals were enrolled as healthy controls. A total of 12 cases and 10 control subjects were included in this cohort.
Study duration
This study was six months (from July 1, 2021, to December 31, 2021).
Case selection
Consecutive cases eligible and willing for the study were included in the study. Control subjects were selected from the hospital staff and relatives of patients.
Ethical considerations
The study was approved by the Institutional Ethics Committee (Ethics Committee Approval Number NIMH/DO/IEC [BS&NS DIV]/2021-22) and was conducted according to the ethical principles for medical research involving human subjects in the Declaration of Helsinki and informed consent was taken from patients and subjects.
Variables, data source, and measurements
Structured questionnaires were used to collect data. The subjects were clinically evaluated by a senior neurologist before inclusion in the study. Demographic data, clinical data, and imaging findings (where available) were recorded. Patients with body mass index (BMI) above 25 g/m2 were categorized as obese (as per the World Health Organization expert group recommendations for people residing in the Asia Pacific region). Carotid ultrasonography was performed using the Philips Affiniti 50 Ultrasound (US) system. Two-dimensional (2D) B-mode sonography, including CIMT measurement, color, and Doppler sonography, was performed using the linear probe (L12-3 MHz). 3D sonography was performed using the volume probe (VL 13-5 MHz). CIMT was measured over the proximal, middle, and distal common carotid artery (CCA). Distal CCA measurements were done at >10 mm distance from the carotid bulb. Although the consensus is to measure CIMT in the distal CCA at least 5 mm proximal to the carotid bifurcation, different authors have used different protocols.[17,18] Far wall CIMT measurements were done in the longitudinal view (anterior or lateral approach), where the carotid vessel wall was free from plaque, and the intima-media layers were clearly visualized. The CIMT was measured by the US system’s vascular plaque quantification (VPQ) program, which provided an automated measurement of a 10 mm segment [Figure 1]. When necessary, the measurement box was repositioned for 100% success (good visualization of intima-media over a 10 mm segment). CIMT measurement was done in both patients and healthy subjects. The results were expressed in two ways, namely, average CIMT (mean of proximal, mid, and distal CCA CIMT) and distal CCA CIMT.
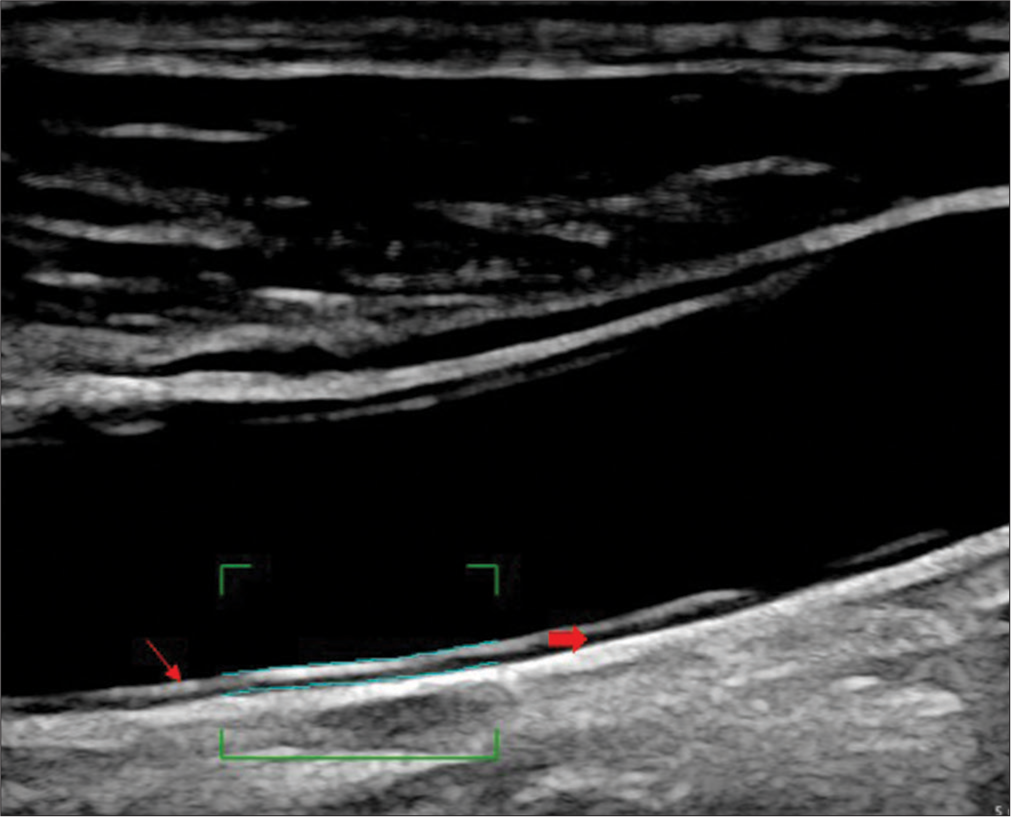
- Measurement of carotid intima-media thickness (CIMT) in distal wall of the common carotid artery (line arrow shows the tunica adventitia and block arrow shows the tunica intima-media, wavy lines within the bracket indicate automated CIMT measurement).
A routine 2D US examination was done to ascertain the presence of carotid atheromatous plaques and to record the number of plaques in each arterial segment. The arterial segments studied include the proximal, mid and distal CCA, external carotid artery and proximal, and mid and distal internal carotid artery (ICA)on either side. Plaque thickness was measured using manual calipers extending from the outer border of the intima up to the maximum protuberance of the plaque into the lumen.[18] Maximum plaque thickness was measured. Plaque area was ascertained using the ellipse tool in 2D US. Qualitative plaque characteristics, including calcification, homogeneity, and ulceration, were noted. Plaques were classified according to the Grey-Weale classification: class I – uniformly reduced echogenicity; class II – heterogeneous texture, mainly hypoechoic; class III – heterogeneous texture, mainly hyperechoic; class IV – uniformly increased echogenicity; and class V – unclassified or calcified plaques.[19] Unstable plaques were defined as plaques with one or more of the following unstable features – heterogeneity, echolucency, surface irregularity, and ulceration. Symptomatic plaques were defined as plaques with a history of TIA or stroke in the same vascular territory.
A linear volume probe was used to obtain 3D images of the distal CCA, carotid bulb, carotid bifurcation, proximal ICA, and any other areas where plaque was suspected. After optimization of 2D image settings, the volume probe was placed in a transverse orientation (probe pointer directed to the right side) over the area of interest, and an automated 3D sweep was performed. The probe was held still, and the patient was requested to remain stationary during the sweep to minimize motion artifact. The 3D images thus obtained were saved in the US system for further analysis.
3D vessel images were viewed using two software programs, namely, GI3DQ and VPQ. GI3DQ (general 3D image viewing program) was used for viewing the region of interest covered in the 3D sweep in three dimensions: Transverse, longitudinal, and coronal. Using these, the approximate location and orientation and plaque characteristics were studied. A detailed plaque analysis was performed using VPQ mode. In the VPQ mode, the entire region of interest appeared in the transverse orientation, divided into thin sections, from caudal to cranial level. An automated program for the calculation of plaque volume was built into the VPQ mode. For this, three sections had to be tagged. Initially, the section just caudal to the beginning of the plaque (plaque-free section) was identified and designated as the “start of plaque.” The outer wall of the vessel was manually drawn using the “ellipse tool” or, in case of the irregular configuration of the vessel (as in the carotid bulb or bifurcation), using the “spline tool.” The machine automatically drew the inner wall (lumenintima interface) [Figure 2]. On double-clicking within the vessel wall, the machine detected the presence or absence of plaque (based on a change in echogenicity). The examiner could also manually indicate the absence of plaque using the “No plaque” tab. Subsequently, the transverse section just cranial to the end of the plaque (plaque-free section) was identified and designated as the “end of plaque.” Next, the sections between the “start of plaque” and “end of plaque” were visually studied. The section showing the maximal reduction in the lumen due to obstruction by plaque was identified and designated as the “key frame.” Subsequently, the plaque was delineated by double-clicking within the vessel’s lumen. If the plaque delineation did not tally with the visual inspection finding, the same could be adjusted by manually dragging the plaque borders, marking additional key frames, or changing the sensitivity of plaque detection (a software setting). Once the plaque borders were delineated correctly, the software calculated plaque volume. Maximum CSA reduction by plaque, a 3D imaging measure of luminal occlusion by plaque, was studied with VPQ software.
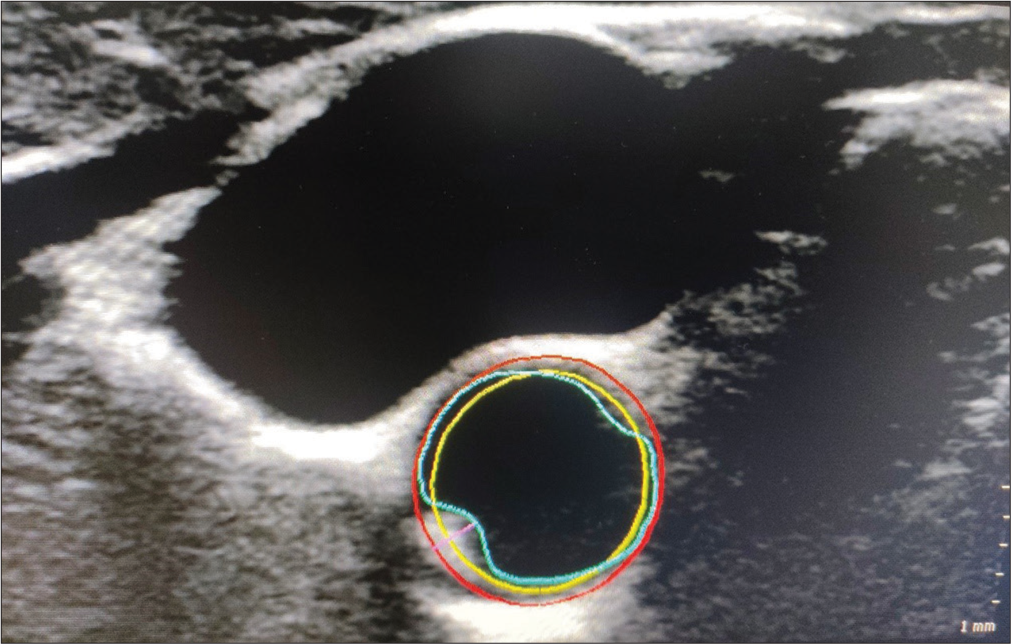
- Carotid plaque detection in vascular plaque quantification mode using key frame. (Red: Outer vessel wall, Yellow: Inner vessel wall, Blue: Lumen-intima interface, Pink: Plaque thickness).
Statistical analysis
The data was entered into the Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics 27.0.1.0) software. Quantitative variables were expressed as mean ± standard deviation (SD). Qualitative variables were expressed as proportions or percentages. Hypotheses were tested using Student’s t-test for quantitative variables and Chi-square test for qualitative variables. P-value below 0.05 was considered significant for all statistical tests.
RESULTS
Although 24 patients met the inclusion criteria, two had to be excluded due to incomplete carotid sonography (as the patients were restless). There was a slight male preponderance, with 66.7% of patients and 60% of controls being male subjects. The anthropometric features [Table 1] and risk factor profile [Table 2] of the subjects are summarized below:
| Height (cm) | Weight (kg) | BMI (kg/m2) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Patients (n=12) | 159.2 | 6.7 | 66.4 | 15.7 | 25.9 | 5.1 |
| Healthy Subjects (n=10) | 160.2 | 10.5 | 59.6 | 7.3 | 23.3 | 3.1 |
SD: Standard deviation, BMI: Body mass index.
| Smoking | Diabetes Mellitus | Hypertension | Dyslipidemia | Cardiac disease | Hyperhomocystinemia | |
|---|---|---|---|---|---|---|
| Patients (n=12) | 41.7% | 8.3% | 41.7% | 16.7% | 8.3% | 16.7% |
| Healthy subjects (n=10) | 10.0% | 30.0% | 10.0% | 10.0% | 0% | 0% |
Among the 12 patients, 10 had a history of stroke, and 2 had a history of TIAs. Patients with all degrees of stroke severity were included in the study. CIMT values were similar in patients and healthy subjects for three site measurements for CCA (patients 0.65 ± 0.10 mm; healthy subjects 0.62 ± 0.06 mm). For distal CCA alone, CIMT was slightly higher on the right side (patients 0.72 mm on the right side and 0.59 mm on the left side; healthy subjects 0.57 mm on the right side and 0.64 mm on the left side). However, this was statistically insignificant. There was a good correlation between right distal CIMT and right average CIMT (k = 0.748, P < 0.001) as well as between left distal CIMT and left average CIMT (k = 0.783, P < 0.001). The mean CIMT in the right CCA was 0.66 mm in obese subjects and 0.58 mm in non-obese subjects (P = 0.073), while that in the left CCA was 0.69 mm in obese subjects and 0.61 mm in non-obese subjects (P = 0.059).
A total of 18 plaques were present among the 22 subjects of the study (right 12, left 6). Nine patients had single plaques, while three had multiple plaques. Two patients had floating thrombi in the CCA. Eleven plaques were located in the CCA, of which three extended distally to the carotid bulb, and one extended up to the ICA. Five plaques were located in the carotid bulb and two were located in the ICA. Eight plaques were symptomatic, and eight were asymptomatic (healthy subjects or plaques in patients with stroke in another vascular territory). Of the 16 plaques studied (excluding the floating thrombi), one belonged to Class 1, two to Class 2, five to Class 3, five to Class 4, and three to Class 5, as per the Grey-Weale classification.[11]
Seven plaques were heterogeneous, while nine were homogenous. Heterogeneity was slightly higher in symptomatic (50%) than in asymptomatic (37.5%). A representative carotid plaque is shown in Figure 3. Plaque surface was irregular in five plaques and smooth in 11 plaques. Surface irregularity was also more frequent in symptomatic (50%) than asymptomatic plaques (12.5 %). Plaque calcification was more common in asymptomatic plaques (25%) than in symptomatic plaques (12.5%). Plaque ulceration was observed infrequently in one of the symptomatic plaques and none of the asymptomatic plaques. Based on the features of the plaque (any one of echolucency, heterogeneous echogenicity, and surface irregularity), ten plaques were categorized as unstable and six plaques as stable plaques. Seven of ten unstable plaques were symptomatic whereas only one of six stable plaques were symptomatic. Plaque heterogeneity, irregular plaque surface, and ulceration were more common in symptomatic carotid plaques. Plaque thickness was higher in patients (3.22 ± 0.95 mm) than in healthy subjects (3.0 ± 2.0 mm). Maximum plaque thickness in symptomatic and asymptomatic plaques was 3.68 ± 0.78 mm and 2.68 ± 1.23 mm, respectively. However, this difference was not statistically significant (P = 0.071).
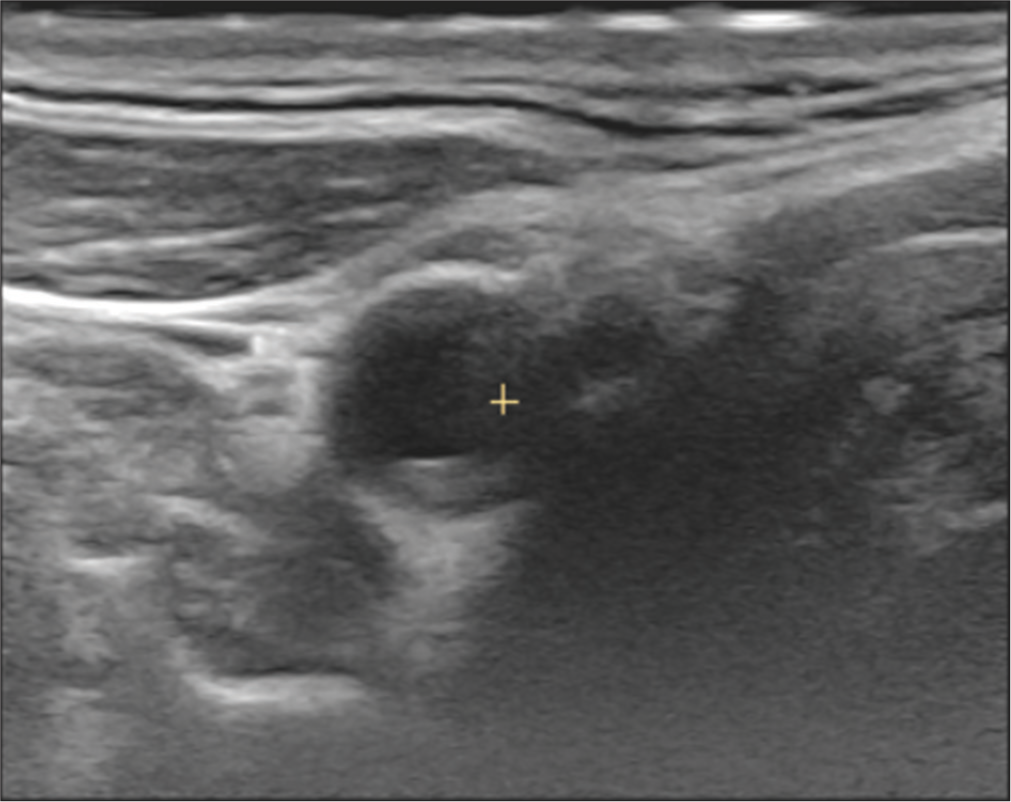
- 3D cross-sectional image of carotid bifurcation showing a smooth plaque with mixed echogenicity.
For 3D US examination, 18 plaques were analyzed. Mean plaque volume among patients was 179.82 ± 310.3 mm3 (Range 11–676 mm3) and in healthy subjects 56.75 ± 69.6 mm3 (range 4–212 mm3). The mean plaque volume in symptomatic plaques was 145.00 ± 85.3 mm3, and in asymptomatic plaques was 129.12 ± 225.6 mm3.
The mean maximum plaque area was higher in patients (18.29 ± 13.8 mm2) than in healthy subjects (11.80 ± 14.2 mm2) and symptomatic plaques (22.03 ± 15.7 mm2) as compared to asymptomatic plaques (12.12 ± 10.3 mm2), though this was not statistically significant. The CSA reduction by 3D sonography was comparable to the degree of stenosis estimated by 2D Doppler criteria in all 18 plaques.
DISCUSSION
Twelve patients with stroke or TIA and ten healthy individuals underwent 2D Doppler followed by 3D carotid sonography to assess their qualitative and quantitative plaque characteristics. Both groups were relatively well matched in terms of age. The mean age of patients was 46.00 years (SD 9.84), and that of healthy controls was 49.90 years (SD 7.92). In a previous large study on carotid plaque morphology from the same state (Karnataka), the mean age of patients was 56.4 years, and that of healthy subjects was 55.2 years.[20] In another similar series from Wardha, India, the mean age was 61 years.[21] The relatively younger age of patients in our study could be due to referral bias, as the study was done in a referral center where there may be an over-representation of younger patients and those with unusual stroke etiologies.
Like most of the previous studies on stroke, we also found a male preponderance.[17,21] The higher prevalence of vascular risk factors, especially smoking, and the better access to health care in the male population may be responsible for this.
Although our patients with stroke showed a higher BMI, this difference was statistically insignificant. Singh et al. reported a mean BMI of 30.25kg/m2 among stroke patients.[22] A Chinese series reported a mean BMI of 23.8 kg/m2 in ischemic stroke patients.[23] These differences in BMI of stroke patients may be related to the difference in etiology of stroke as well as the difference in population prevalence of obesity in various regions. In a population-based study from Telangana (India), the main risk factors for stroke were smoking (54%), hypertension (67%), and diabetes mellitus (57%).[24]
The correlation between distal CCA CIMT and average CIMT on both the right and left sides indicates that measurement of distal CCA CIMT alone, which is less time-consuming, may give an adequate measure of the mean CIMT. A previous study from Sri Lanka showed that composite CIMT was more predictive of CAD than segment-specific CIMT scores.[25] In our study, we found no significant difference in the mean CIMT of patients (0.65 ± 0.10 mm) and controls (0.62 ± 0.06 mm). A West Indian study reported that the mean CIMT in patients with ischemic stroke was 0.83 mm (SD: 0.14 mm).[17] However, the method of calculation of CIMT was based on the average of measurements of the near and far walls of the CCA, carotid bulb, and ICA. Sahoo et al. measured CIMT in the distal CCA and reported a mean CIMT of 0.798 mm in patients with stroke and 0.60 mm in healthy persons.[26] In a series from Bangalore, the mean CIMT in patients with stroke was 0.912 mm.[20] The lower CIMT value in our patients may be related to less severe atherosclerotic process, lower frequency or severity of CAD, or small sample size. However, the CIMT in the current control subjects was similar to the previous studies.[12]
A study by Sahoo et al. reported that CIMT in patients with plaque was significantly higher than in those without plaque.[26] Our study also indicates that CIMT was higher in patients with plaques, though statistical significance was not attained. CIMT was higher on both sides in obese subjects as compared to others. A series from Uttar Pradesh (2013) showed a significant correlation between obesity and CIMT.[22]
A recent study from Norway, where 26 carotid plaques were analyzed, noted no significant relationship between plaque composition and surface and symptomatic plaques.[27] Studies have shown that patients with carotid plaque surface irregularities and ulcers are more likely to have a history of MI, stroke, fatal acute MI, and sudden cardiac deaths.[28] Our study showed that plaque heterogeneity, surface irregularity, and ulceration were commoner in symptomatic plaques. However, a larger sample size may be required to further characterize these features’ role in assessing plaque vulnerability. Tissue characterization from 3D US may improve risk stratification, monitoring for the effectiveness of management, and selection of patients for carotid revascularization.[3]
In our study, the mean plaque volume among patients was 179.82 ± 310.3 mm3, and in healthy subjects, 56.75 ± 69.6 mm3. In a recent study based on 3D US, the plaque volume in subjects undergoing carotid endarterectomy was around 1000 mm3, which was similar to that ascertained from the endarterectomy specimen. The plaque volume reported was much higher as the study included patients with severe carotid stenosis.[29] In this study, carotid plaque was increased in the 270 symptomatic patients compared to the 69 patients with no symptoms of cerebral ischemia (0.97 [0.43] versus 0.74 [0.41] cm3; P < 0•001). Another study showed that symptomatic plaques had statistically significant increases in average CSA (541.52 mm2) and Lumen volume (394.64 mm3) when compared with asymptomatic ones (371.18 mm2 and 245.92 mm3, respectively) and can serve to accurately assess the risk of stroke in patients with carotid stenosis, independent of NASCET criteria stratification.[30]
The strength of our study is that there are no previous studies on plaque volume assessment in the literature, except for studies on interobserver agreement. Limitations include the fact that the study is underpowered to draw conclusions about features of plaque instability. Hence, further studies with larger sample sizes are required to confirm these observations.
CONCLUSION
A 3D carotid US is an accurate niche technique for monitoring the progression of carotid atherosclerosis. It allows a fast and reproducible volumetric quantification of disease and aids in monitoring preventive strategies and follow-up. Larger numbers are required to confirm the IMT, plaque surface area, and plaque volume in stroke subjects compared to healthy subjects in the Indian scenario.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: KGK: Study conception, data collection, data analysis, and manuscript preparation; VK: study conception, data collection, data analysis, and manuscript editing; SR: data analysis and manuscript editing, RK: data analysis and manuscript editing, AHR: data analysis.
Ethical approval
The research/study approved by the Institutional Review Board at the National Institute of Mental Health and Neurosciences, number NIMH/DO/IEC (BS&NS DIV)/2021-22, dated August 10, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Unstable carotid artery plaque: New insights and controversies in diagnostics and treatment. Croat Med J. 2016;57:311-20.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: A 10-year follow-up of 6584 men and women: The Tromsø Study. Stroke. 2011;42:972-8.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional echographic evaluation of carotid artery disease. J Cardiovasc Echogr. 2018;28:218-27.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of atherosclerotic plaques in carotid arteries by three-dimensional ultrasound. Br J Radiol. 1994;67:672-8.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional ultrasound of carotid atherosclerosis: Semiautomated segmentation using a level set-based method. Med Phys. 2011;38:2479-93.
- [CrossRef] [PubMed] [Google Scholar]
- Single sweep three-dimensional carotid ultrasound: Reproducibility in plaque and artery volume measurements. Atherosclerosis. 2014;232:397-402.
- [CrossRef] [PubMed] [Google Scholar]
- Can carotid bulb plaque assessment rule out significant coronary artery disease? A comparison of plaque quantification by two-and three-dimensional ultrasound. J Am Soc Echocardiogr. 2013;26:86-95.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional ultrasound quantification of intensive statin treatment of carotid atherosclerosis. Ultrasound Med Biol. 2009;35:1763-72.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis. 2011;219:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of ultrasound measurements to assess carotid atherosclerosis development in subjects with and without type 2 diabetes. Cardiovasc Ultrasound. 2005;3:15.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv Cardiovasc Dis. 2007;1:97-106.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal ultrasound evaluation of carotid atherosclerosis in one, two and three dimensions. Ultrasound Med Biol. 2009;35:367-75.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of atorvastatin on carotid atherosclerotic plaques: A randomized trial for quantitative tissue characterization of carotid atherosclerotic plaques with integrated backscatter ultrasound. Cerebrovasc Dis. 2009;28:417-24.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid plaque burden as a measure of subclinical atherosclerosis: Comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681-9.
- [CrossRef] [PubMed] [Google Scholar]
- A three-dimensional ultrasonographic quantitative analysis of non-ulcerated carotid plaque morphology in symptomatic and asymptomatic carotid stenosis. Atherosclerosis. 2008;198:129-35.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of 3-dimensional ultrasound imaging to evaluate carotid artery stenosis: Comparison with magnetic resonance angiography. J Stroke Cerebrovasc Dis. 2015;24:148-53.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid intima-media thickness and apolipoproteins in patients of ischemic stroke in a rural hospital setting in central India: A cross-sectional study. J Neurosci Rural Pract. 2012;3:21-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011): An update on behalf of the advisory board of the 3rd and 4th watching the risk symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2012;34:290-6.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino). 1988;29:676-81.
- [Google Scholar]
- Relevance of carotid intima-media thickness and plaque morphology in the risk assessment of patients with acute ischemic cerebral infarcts: A case-control study of large series from a single centre. J Med Ultrasound. 2019;28:29-34.
- [CrossRef] [PubMed] [Google Scholar]
- Age-wise association of carotid intima-media thickness in ischemic stroke. Ann Neurosci. 2017;24:5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid intima-media thickness as a reflection of generalized atherosclerosis is related to body mass index in ischemic stroke patients. N Am J Med Sci. 2013;5:228-34.
- [CrossRef] [PubMed] [Google Scholar]
- Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci Rep. 2017;7:2507.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for stroke in rural population of Telangana state of India, an unmatched case control study. J Neurosci Rural Pract. 2020;11:448-53.
- [CrossRef] [PubMed] [Google Scholar]
- Composite carotid intima-media thickness as a risk predictor of coronary heart disease in a selected population in Sri Lanka. PLoS One. 2022;17:e0271986.
- [CrossRef] [PubMed] [Google Scholar]
- Common carotid intima-media thickness in acute ischemic stroke: A case-control study. Neurol India. 2009;57:627-30.
- [CrossRef] [PubMed] [Google Scholar]
- The course of carotid plaque vulnerability assessed by advanced neurosonology. Front Neurol. 2021;12:702657.
- [CrossRef] [PubMed] [Google Scholar]
- Symptomatic carotid atherosclerotic disease: Correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46:182-9.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid plaque volume in patients undergoing carotid endarterectomy. Br J Surg. 2018;105:262-9.
- [CrossRef] [PubMed] [Google Scholar]
- Plaque contact surface area and lumen volume predict stroke risk in extracranial carotid artery stenosis. J Vasc Surg. 2022;76:482-8.
- [CrossRef] [PubMed] [Google Scholar]






