Translate this page into:
Assessment of memory deficits in psychiatric disorders: A systematic literature review
*Corresponding author: Dr. Roshan Fakirchand Sutar, Assistant Professor, Department of Psychiatry, All India Institute of Medical Sciences Bhopal. Madhya Pradesh - 462 020, India. roshan.psy@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Kushwaha A, Basera DS, Kumari S, Sutar RF, Singh V, Das S, et al. Assessment of memory deficits in psychiatric disorders: A systematic literature review. J Neurosci Rural Pract. 2024;15:182-93. doi: 10.25259/JNRP_456_2023
Abstract
Memory deficits are observed across psychiatric disorders ranging from the prodrome of psychosis to common mental disorders such as anxiety, depression, and dissociative disorders. Memory deficits among patients recovering from psychiatric disorders could be directly related to the primary illness or secondary to the adverse effect of a treatment such as Electroconvulsive Therapy (ECT). The trouble in the meaningful integration of working-memory and episodic memory is the most commonly affected domain that requires routine assessments. An update on the recent trends of methods of assessment of memory deficits is the first step towards understanding and correcting these deficits to target optimum recovery. A systematic literature search was conducted from October 2018 to October 2022 to review the recent methods of assessment of memory deficits in psychiatric disorders. The definition of ‘Memory deficit’ was operationalized as ‘selective processes of memory, commonly required for activities of daily living, and affected among psychiatric disorders resulting in subjective distress and dysfunction’. We included 110 studies, most of them being conducted in western countries on patients with schizophrenia. Other disorders included dementia and mild cognitive impairment. Brief Assessment of Cognition in Schizophrenia, Cambridge Automated Neuropsychological Test Battery, California Verbal Learning Test, Trail Making Test Part A and B, Rey Auditory Verbal Learning Test, Wechsler Memory Scale, Wechsler Adults Intelligence Scale-IV were the most common neuropsychological assessments used. Mini-Mental State Examination and Montreal Cognitive Assessment were the most common bedside assessment tools used while Squire Subjective Memory Questionnaire was commonly used to measure ECT-related memory deficits. The review highlights the recent developments in the field of assessment of memory deficits in psychiatric disorders. Findings recommend and emphasize routine assessment of memory deficits among psychiatric disorders in developing countries especially severe mental illnesses. It remains interesting to see the role of standardized assessments in diagnostic systems given more than a decade of research on memory deficits in psychiatric disorders.
Keywords
Memory deficits
Cognitive
Recent memory
Working memory
Assessment
psychiatry
Neuropsychological assessment
INTRODUCTION
Memory is defined as the faculty of the brain essential for encoding, storing, and retrieving information.[1] Memory is an integral part of healthy living and most psychiatric disorders including depression, anxiety, psychotic illnesses, and prodromal syndromes are known to have variable levels of impairments in memory.[2] The forgetfulness brought about by the functional or organic nature of an illness has great implications for recovery from an illness and has widely been appreciated in the literature. Most commonly appreciated deficits in memory are found in episodic, autobiographical, and working memory (WM) domains among psychiatric disorders. Furthermore, immediate and recent recall is probably the most commonly performed bedside test during an examination of a psychiatry case, which also forms an integral part of the mental status examination. The WM is relevant in psychiatry because it forms a connection to the integration of emotional aspects with cognitive tasks besides forming a channel to long-term memory.[3] In addition, it also facilitates the task-completion supplemented by other executive functions of the brain. Under stressful conditions, a dynamic change happens in the memory throughout life. This part of memory refers to the ability to hold the stimuli “online” for a short time then either use it directly after a short delay or process or manipulate it mentally to solve cognitive and behavioral tasks.[4] The WM involves active rehearsing, processing, and manipulation of information that depends on the function of the prefrontal cortex. It is well recognized that long-term memory is retained while short-term memory is vulnerable to organic brain damage.[5] Certain memories may not be verbal, and they are often procedural which could be implicit (limbic) and explicit (hippocampus).[6] Again autobiographical memory summates the subjective details of personal experience, which are subsumed under episodic memory. Semantic memory is more concerned about recollecting facts of the matter such as the capital of the city.[7] Therefore, impairment in episodic or WM could cause trouble in the meaningful integration of our existence. Psychiatric disorders are state phenomena; therefore, the impairment in memory could be transient and modulated by dysfunctional neural connectivity. Meaningful recovery is largely dependent on the assessment and management of memory impairment.[8,9] The assessment of memory deficits could be carried out through bedside testing, structured scales or detailed neuropsychological assessments. It is known that different psychiatric disorders could have impairments in specific domains of memory.[9] Various methods of assessment of memory deficits have been widely used across the world, and it would be useful to look at the current trend and development in the field of neuropsychiatry in terms of assessment across the world. It would also be interesting to understand the cross-cultural use of similar tests across different disorders, along with the use of standardized tools such as Mini-mental state examination (MMSE). Through this review, we aimed to look at the current trends in memory assessments, tools used, and domains studied in psychiatric disorders across the world in the past 5 years.
METHODS
We operationalized the memory deficits in psychiatric disorders as “selective processes of memory that are most commonly affected during the illness or treatment of an illness with development of subjective distress and dysfunction in daily activities.” The operational definition included the assessment of verbal memory, visual memory, WM, episodic memory, autobiographical memory, short-term memory, declarative memory, logical memory, facial recognition, and visuospatial memory. The definition of memory deficits was limited to the above domains, and we excluded the assessments of other executive domains and complex cognitive processes such as cognitive flexibility, set-shifting, response inhibition, and processing speed.
Search strategy and terms
A systematic literature search of the following databases was conducted on November 1, 2022, by two independent authors (RS and AK) on PubMed and Google scholar. In addition, the reference lists of the pertinent literature were screened for relevant literature. Literature was searched using the search terms: Memory AND psychiatric illness, memory deficit AND psychiatry AND assessment, memory deficit AND psychiatric disorders, memory impairment AND schizophrenia AND assessment, memory impairment AND depression AND assessment, memory impairment AND dementia AND assessment, memory AND depression AND assessment, memory AND anxiety AND assessment, and memory AND bipolar disorder AND assessment. AK and RS screened the articles using the inclusion/exclusion criteria with assistance from DB.
Eligibility criteria for studies
We included all the observational and interventional studies (including cross-sectional, case–control, and cohort), and published protocols of such studies in the past 5 years that mentioned the methods of assessment of memory deficits in any psychiatric disorders published in the English language from October 2017 to October 2022. We included mild cognitive impairment (MCI) and dementia as psychiatric disorders in this review due to extensive evidence of behavioral disturbances found in them.
TYPE OF PARTICIPANTS
We included studies with subjects of either gender or any age. We excluded animal studies and studies on healthy subjects, healthy geriatric populations, various cancers, Parkinson’s, multiple sclerosis, stroke, road traffic accidents, human immunodeficiency virus, chronic obstructive pulmonary disease, COVID, diabetes mellitus, systemic lupus erythematous, cancer-survivors, domestic violence, and subjects with cochlear implants or any other medical conditions.
TYPE OF OUTCOME MEASURES
Primary outcome
Types of assessment methods used for identifying memory deficits in psychiatric disorders and most commonly studied psychiatric disorders.
Secondary outcome
Most commonly tested domains of memory.
Selection process
RS and AK carried out the preliminary search. Author AK and RS read the abstracts and extracted the relevant articles for full text in Microsoft Excel. Any duplicate citations were removed by DB. Color coding was used for any discrepancies with the resolution by the involvement of DB.
Data collection process
Studies reporting the assessment methods of any type were further read for mention of details by two independent reviewers DB and RS. RS extracted the data into a table in Microsoft Word and then did another full-text screening of all the articles. RS and AK extracted the raw data from each article and put it into another table for further analysis.
Data items
Studies were read in detail and data extracted included author, year of publication, type of study, country of research, assessment method used, psychiatric disorder studied, and domains of memory assessed.
RESULTS
Result of the study selection
A total of 1699 studies were identified from the databases. After the removal of 108 duplicate studies, 1591 studies were reviewed. The 1444 studies were excluded because assessment methods of interest were not there, animal studies or not relevant to the review objective or were not in English. After the full-text screening, 147 studies were, further, screened for eligibility and 110 were included in the review as shown in Figure 1.
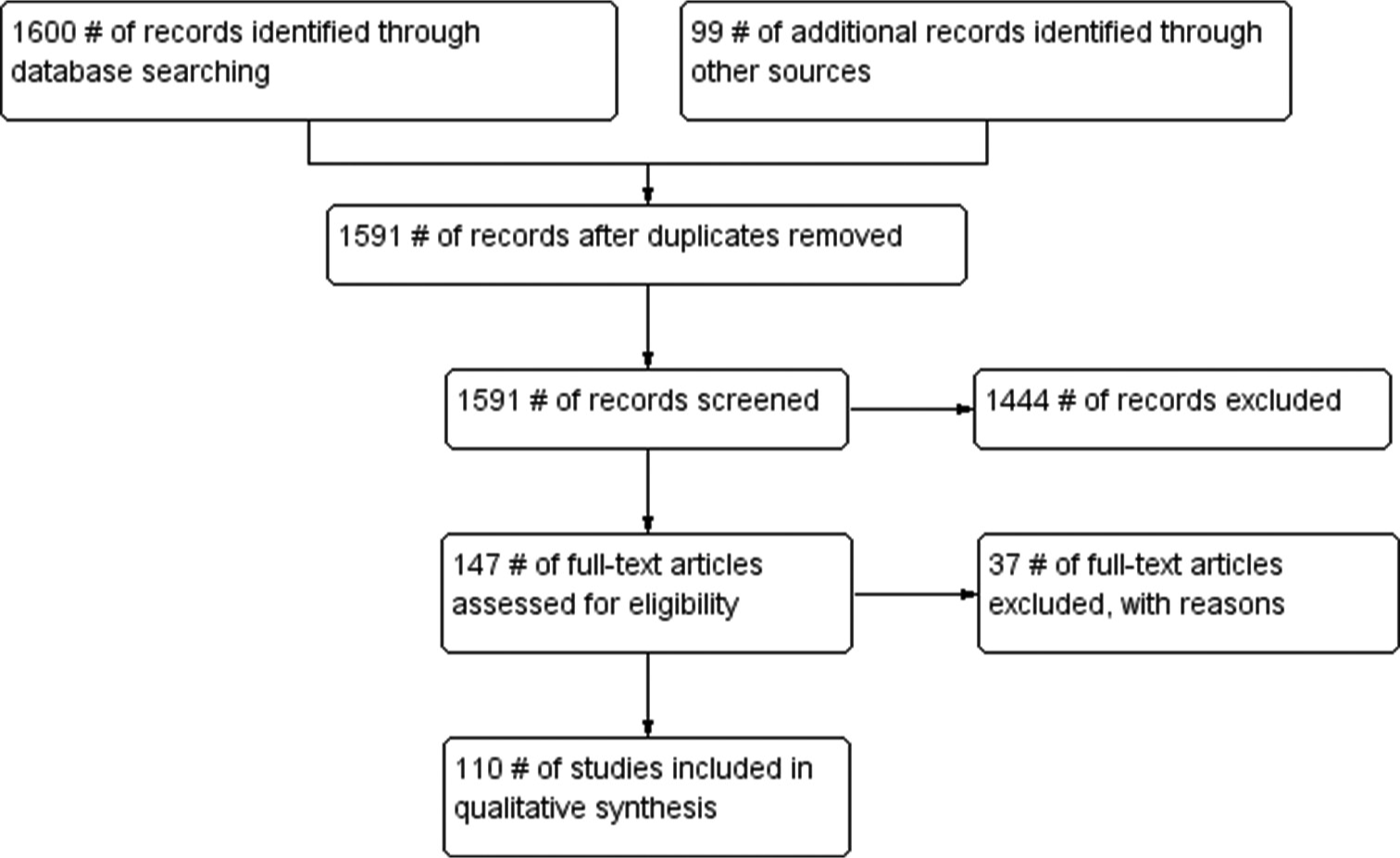
- PRISMA flow diagram.
Study-site
The majority of the studies (n = 30) were from Europe (particularly from Spain and Sweden) followed by 26 from the USA. There were 17 studies from the Asian continent (mostly from China and South Korea) and six studies each from India and Canada. Two studies were each from Mexico, Israel, and Australia, and ten studies were multicentric.
Type of studies
Most studies were cross-sectional and observational, and some were retrospective chart reviews. Few were prospective two-staged interventional studies. Very few were interventional randomized controlled trials for studying the effectiveness of interventions for improving memory deficits or interventions that lead to memory deficits.
Studies included under review
We included 55 studies on psychiatric disorders with predominant memory impairment such as schizophrenia, bipolar disorder, substance use disorder, and depression, as detailed in [Supplementary Table 1],[10-64] whereas minor impairment in memory (17 papers) included suicide attempters, anxiety disorders, fibromyalgia, eating disorders, obsessive compulsive disorder, post-traumatic stress disorder (PTSD), dissociative disorder, attention deficit hyperactivity disorder, Autism spectrum disorder, and borderline personality disorder as shown in Supplementary Table 2.[65-81]
Studies focusing on neuropsychiatric disorders in the review
We found 29 studies[38,82-109] on neuropsychiatric disorders with major memory impairments such as dementia, MCI, and vascular events while nine studies on memory deficits related to electroconvulsive therapy (ECT).[72,110-117] These details are shown in Supplementary Tables 3 and 4, respectively.
Domains of memory were included for the review
Registration and recall are the predominant domains highlighted in the review. The details assessed in various studies included fluency, planning and perseveration, inattention, sustained attention and vigilance, declarative memory, immediate and delayed verbal memory performance, memory for complex shapes, WM, visual delayed recall, rapid visual information processing, verbal memory, attention and concentration, recent memory, language, visual-constructional ability, orientation, calculation, semantic fluency, logical memory, verbal learning, verbal IQ, auditory tone matching, phonological awareness (non-word) reading fluency, verbal memory, working semantic fluency, letter fluency, verbal fluency language, calculation, WM, attention, and short-term memory. Commonly used tests were reported at various studies[10-64] and are shown in Supplementary Table 1.
Highlights of the common tests used in psychiatry in the past 5 years
We reviewed the commonly used neuropsychological tests for memory assessments[65-81] that are being summarized in Table 1, whereas bedside clinical scales included MMSE, Delta MSE, Baylor profound MSE, Montreal cognitive assessment (MoCA), and cognitive failure questionnaire. These tests simulate the situations of daily-life activities targeting specific events and would require a recall of specific details in the encoding context. It appears necessary to identify flaws during the various stages of information encoding, consolidation, and retrieval.
| Heading 1 | Heading 2 |
|---|---|
| TMT-A and TMT-B | This is one of the most popular neuropsychological tests and is included in most test batteries. The TMT provides information on visual search and working memory along with scanning, speed of processing, mental flexibility, and executive functions. This test can be administered within the age range of 8–79 years, 11 months and it takes 5–15 min to administer. |
| WMS | This is a neuropsychological test designed to measure different memory functions in a person. Anyone aged 16–90 years is eligible to take this test. The current version is the fourth edition (WMS-IV) which was published in 2009 and was designed to be used with the WAIS-IV. A person’s performance is reported as five index scores: Auditory memory, visual memory, visual working memory, immediate memory, and delayed memory. WMS requires 45–60 min for administration. |
| RAVLT | The RAVLT is a neuropsychological tool used to assess cognitive functions such as attention, verbal learning, and short-term memory. |
| CVLT | This is a comprehensive, detailed assessment of verbal learning, and memory deficits in older adolescents and adults. Anyone aged 16–89 years is eligible to take this test. This test takes 30 min for the administration. |
| RBANS | This test was developed for the dual purposes of identifying and characterizing abnormal cognitive decline in older adults and as a neuropsychological screening battery for younger patients. The entire battery takes<30 min to administer and yields scaled scores for five cognitive domains. |
| BACS | This is a newly developed instrument that assesses the aspects of cognition found to be most impaired and most strongly correlated with outcomes in patients with schizophrenia. The BACS requires<35 min to complete in patients with schizophrenia |
| AMI | This is a semi-structured interview designed to assess memory for autobiographical information, impairment of which is often indicative of retrograde amnesia (inability to recall previously learned information or past events) and potentially associated with a variety of neurological and psychiatric disorders. |
| HVLT-R | This test was used to assess acquisition and delayed recall. A new test for verbal learning and memory, the test consists of three trials of free-recall of a 12-item. The performance of patients with Alzheimer’s disease and chronic amnesia is described. The test is likely to be useful in patients too impaired for more comprehensive memory assessments and where repeated testing is necessary. |
| MoCA | This is a test used by healthcare providers to evaluate people with memory loss or other symptoms of cognitive decline. It can help identify those at risk for developing Alzheimer’s disease and other forms of dementia. It is also used as a screening tool for conditions such as Parkinson’s disease, brain tumors, substance abuse, and head trauma. The MoCA contains 30 questions and takes around 10–12 min to complete. It is a useful screening test, but it needs to be considered alongside the results of other tests to confirm a diagnosis. |
| ROCF test | This test is a commonly used neuropsychological assessment tool. It is widely used to assess the visuoconstructional ability and visual memory of neuropsychiatric disorders, including copying and recall tests. By drawing the complex figure, the functional decline of a patient in multiple cognitive dimensions can be assessed, including attention and concentration, fine-motor coordination, visuospatial perception, non-verbal memory, planning and organization, and spatial orientation |
| MCCB | This recently developed tool measures cognition in individuals diagnosed with schizophrenia and related disorders. The test can be administered to an individual with the age range: of 20–59 years and it takes time 60–90 min for the administration. Consists of ten individually administered tests that measure cognitive performance in seven domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning, problem-solving, and social cognition. |
BACS: Brief assessment of cognition in schizophrenia, TMT: Trail making test, MATRICS: Measurement and treatment research to improve cognition in schizophrenia, HVLT-R: Hopkins verbal learning test-revised, CVLT: California verbal learning test, WMS: Wechsler memory scale, RAVLT: Rey auditory verbal learning test, MoCA: Montreal cognitive assessment, WAIS-IV: Wechsler adults intelligence scale-IV, MCCB: MATRICS consensus cognitive battery, ROCF: Rey–Osterrieth complex figure, AMI: Autobiographical memory interview, RBANS: Repeatable battery for the assessment of neuropsychological status
Whereas the subjective reporting of memory difficulties in many patients could be assessed using the Squire subjective memory questionnaire (SSMQ), Memory Assessment Clinic Questionnaire (MAC-Q), memory items from the Comprehensive Psychiatric Rating Scale (CPRS), and subjective memory complaint (SMC) scale. These tests are important as the disparity between subjective and objective memory is common in psychiatric disorders.
In recent years, automated tests have been used widely for research, also known as computerized battery, which includes a variety of assessments of neurocognitive functions and processes such as psychomotor and motor speed, reasoning, and planning abilities, memory and attention, and frontal, temporal, and hippocampal dysfunctions are available. They predominantly include Cambridge Automated Neuropsychological Test Battery, THINC-integrated tool, and Pennsylvania computerized neurocognitive tests, which are being widely employed for research across the disciplines of medicine, psychiatry, rehabilitation, and neurology. Uniformity across the subjects, optimum duration (40–45 min), language independence, standardized nature, and gender neutrality are the core features of these tests. Number of studies in the past five years, assessing the memory deficits in psychiatric disorders are shown in Supplementary Figure 1.
DISCUSSION
The findings of this review reinforce the growing number of studies on the assessment of memory deficits in dementia and MCI followed by schizophrenia. The development in the field of neuropsychology using automated tests for dementia is rapidly growing and has also been replicated in primary psychiatric disorders such as schizophrenia in the past five years. A significant number of studies were reported from the West as compared to the East (more from Europe and USA followed by Japan and Korea). Both, treated as well as untreated, patients with schizophrenia frequently experience difficulties in cognitive domains that contribute toward overall dysfunction[118,119] and brief assessment of cognition in schizophrenia, Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), trail making test part A and B (TMT-A and TMT-B), and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) are the most commonly used assessment methods in the past 5 years. They can capture the most commonly established memory deficits such as WM and verbal and visual memory deficits in an active phase of illness as well as during remission. Most of the studies reported in this review are from the developed world and recognize the impact of assessing memory deficits on functioning, quality of life, treatment adherence, and a prolonged period of recovery.[54] The highlights of the specific type of memory deficits in psychiatric disorders are shown in Table 2.
| S. No. | Disorder/clinical condition | Commonly affected domain | Assessments |
|---|---|---|---|
| 1. | Schizophrenia | Working memory, processing speed, verbal learning memory, and visual learning memory | BACS, MATRICS battery, TMT-A and TMT-B, RBANS, HVLT, and CVLT |
| 2. | Mood disorder | Episodic memory – (particularly poor memory for positive events), working memory. | TMT-A and TMT-B, Stroop test, SCT, SCWT, WMS spatial working memory test, CVLT, RAVLT, continuous performance test, and BVMT |
| 3. | Anxiety disorders | Working memory, registration, and recall, declarative memory particularly in PTSD | SCWT, CFQ, Digit span test, TMT, and WMS |
| 4. | Alcohol dependence | Autobiographical memory and working memory | CANTAB, MoCA, working memory – WAIS-IV, Digit Span test, MMSE, etc. |
| 5. | MCI | SMCs, working memory, and short-term memory | MMSE, MoCA, Tests related to SMCs, CERAD, WMS, etc. |
| 6. | ECT-related memory problems | Short-term memory and autobiographical memory | MMSE, B4ECT-ReCoDe, delta-MMSE, Squire SMC, AMC, and m-CPRS |
BACS: Brief assessment of cognition in schizophrenia, BVMT: Brief visuospatial memory test, CANTAB: Cambridge neuropsychological test automated battery, CERAD-NP: Consortium to establish a register for Alzheimer’s disease-neuropsychological test battery, CVLT: California verbal learning test, CPRS: Comprehensive psychopathology rating scale, ECT: Electroconvulsive therapy, HVLT: Hopkins verbal learning test, MATRICS: Measurement and treatment research to improve cognition in schizophrenia, MCI: Mild cognitive impairment, MMSE: Mini-mental state examination, MoCA: Montreal cognitive assessment, TMT-A and TMT-B: Trail making test part A and B, RAVLT: Rey auditory verbal learning test, RBANS: Repeatable battery for the assessment of neuropsychological status, WMS: Wechsler memory scale, WAIS-IV: Wechsler adults intelligence scale-IV, SCT: Stroop color test, SCWT: Stroop color-word test, CFQ: Cognitive failures questionnaire, PTSD: Post-traumatic stress disorder, SMC: Subjective memory complaint
Studies on common mental disorders such as mood disorders, anxiety disorders, and PTSD have reported deficits in WM and episodic memory using tests such as TMT-A and TMT-B, Stroop test, Stroop color test, and Stroop color-word test, Wechsler’s memory scale, spatial WM (SWM) test, California verbal learning test, Rey Auditory Verbal Learning Test and Continuous Performance Test. Mechanism of stress-induced reversibility of amnesia in dissociative disorders, pseudo dementia in depressive disorders, and flashbulb memory of PTSD requires uniform assessment methods as highlighted in this review.[120] The neurobiological explanation is beyond the scope of this review. Patients undergoing ECT also report memory impairment in areas of short-term and autobiographical memory and MMSE, B4ECT-ReCoDe, delta-MMSE (d-MMSE), Squire SMC, autobiographical memory inventory, and CPRS are commonly used assessment methods in this regard. SMCs, WM impairment, and short-term problems are frequently reported problems in patients with MCI and dementia. In these patients, MMSE, MoCA, tests related to SMCs, Consortium to Establish a Registry for Alzheimer’s Disease, neuropsychological comprehensive batteries, Wechsler’s memory scale, etc., are used for assessment of these problems. This information gives a fair idea to establish stable deficits (depression, schizophrenia, PTSD, and personality), progressive deficits (MCI, dementia, and ECT-induced) or track the improvement by choosing the set of tests for particular disorders.
Because most of the studies are cross-sectional, the consistency of measurement being tested and tests applied should be replicated among longitudinal and interventional studies.
The widely used MMSE provides tentative estimates about a delayed recall given the lesser time between registration and recall while Addenbrooke’s cognitive assessment is one such test to complement the MMSE in resource-prone countries. Most of the selected studies have used a battery of tests for assessments of more than one cognitive function including processing speed, response inhibition, set shifting, and cognitive flexibility; however, this review focused only on the memory deficits in psychiatric disorders, the discussion of other executive functions is beyond the scope of this review.
Further, illiterate patients, patients with dementia, MCI,[121] and patients undergoing ECT frequently present with SMCs, and for them, it is important to use a subjective memory questionnaire periodically to quantify the memory deficits which have been validated as any other standard objective assessment tool.[122-124] The review findings emphasize the regular use of bedside objective measures such as MMSE, HMSE, MoCA, and d-MMSE in suspected subjects with psychiatric illnesses to track the trajectory toward recovery. At the same time, it is recommended to support with the most commonly used subset of neuropsychological tests. The B4ECT-ReCoDe is one such tool developed in India that informs us of differential memory deficits caused by unilateral ECT, ultra-brief pulse ECT, and bifrontal ECT.[124]
In the end, the review brings the latest developments in the past 5 years in terms of the assessment of memory deficits in psychiatric disorders. This review helps the clinician to overcome the difficulties faced during the evaluation of memory deficits and revisit the knowledge to help themselves in medicolegal situations. One-stop availability of these methods of assessment makes it easy for researchers to acknowledge the current updates in the field of neuropsychology in psychiatric disorders including other mood disorders and psychotic disorders.[118] An upcoming self-administered or mobile device-based application to assess memory deficits could also provide good reliability and validity. A more detailed account of such validation should reach universal acceptance and prioritize future research. Although the memory deficits are subtle and transient, they play an important role in the maintenance of the pathological process, relapse, and become a barrier to functional recovery unless assessed on the given tools with adequate address for further recovery.[8] We emphasize the use of these tests routinely to complement the history and MSE findings while understanding their limitations.[125] In addition, protocols should be developed for recognizing the importance of functional neuroimaging to supplement the findings of the tests. For more than a decade of research on cognitive deficits in psychiatric disorders specifically schizophrenia, still no consensus/agreement helped to inform the diagnostic systems in psychiatry.[126]
Translational implications
Understanding different domains of memory and their assessment is important for physicians, who often need to assess WM in children, adults, and the elderly. Apart from organic causes of memory deficits, such as MCI and dementia, the slow deterioration of memory is evident in severe mental illnesses. Clinicians should be able to identify and recognize the signs of WM deficits in persons with mental health conditions, while psychiatrists should be aware of how to plan the assessments and treatment. While these approaches translate the clinical practice, we recognize the importance of developing neuroprotective pharmacotherapeutic agents that could help in improving cognition in psychiatry. To facilitate the understanding and ease the complexity, medical education is also required to emphasize the concepts of different types of memory, such as episodic, semantic, short-term, long-term, and autobiographical, and integrate them horizontally and vertically in the curriculum while residential courses in psychiatry are required to take up such assessments periodically to better translate their knowledge in practice. Although the specificity of memory deficits is not clear, clinical assessment could still find the patterns of deficits in certain phenotypes that can aid the diagnostic system in psychiatry.
Limitations of the study
The review is targeted to identify only memory deficits and not whole neurocognitive deficits encountered among psychiatric disorders because it is one of the most common subjective complaints reported by patients and these deficits are not commonly addressed. We also included the memory deficits encountered in dementia and MCI as these patients frequently encounter psychiatric services. The included selective memory deficits in the review could give us great insight into behavioral management and the tests used for them.
CONCLUSION
The review brings the latest developments in the past five years in terms of the assessment of memory deficits in psychiatric disorders. This review helps the clinician to overcome the difficulties faced during the evaluation of memory deficits and revisit the knowledge to help themselves in medicolegal situations. An upcoming self-administered or mobile device-based application to assess memory deficits could be a future alternative to provide reliable estimates. A more detailed account of such validation should reach universal acceptance and prioritize future research. In addition, protocols should be developed for recognizing the importance of functional neuroimaging to supplement the findings of the tests and they should be encouraged to become a part of the formal diagnostic and prognostic systems in psychiatry.
Authors’ contributions
The contributions of each author should be described. RS: Conceptualization, investigation, writing-original draft, and writing-review and editing. AK: Investigation, writing-original draft, and writing-review and editing. DB: Validation, writing-review and editing, and supervision. All authors read and approved the submitted version.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Memory and brain systems: 1969-2009. J Neurosci. 2009;29:12711-6.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100-11.
- [CrossRef] [PubMed] [Google Scholar]
- Plasticity in the working memory system: Life span changes and response to injury. Neuroscientist. 2018;24:261-76.
- [CrossRef] [PubMed] [Google Scholar]
- What are the differences between long-term, short-term, and working memory? Prog Brain Res. 2008;169:323-38.
- [CrossRef] [PubMed] [Google Scholar]
- The neuroanatomical, neurophysiological and psychological basis of memory: Current models and their origins. Front Pharmacol. 2017;8:438.
- [CrossRef] [PubMed] [Google Scholar]
- Life-span development of visual working memory: When is feature binding difficult? Dev Psychol. 2006;42:1089-102.
- [CrossRef] [PubMed] [Google Scholar]
- Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348-57.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168:213-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive deficits in psychiatric disorders: Current status. Indian J Psychiatry. 2006;48:10-20.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated hair cortisol is associated with childhood maltreatment and cognitive impairment in schizophrenia and in bipolar disorders. Schizophr Res. 2019;213:65-71.
- [CrossRef] [PubMed] [Google Scholar]
- Lowered serum cesium levels in schizophrenia: Association with immune-inflammatory biomarkers and cognitive impairments. Braz J Psychiatry. 2021;43:131-7.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of psychiatric illnesses and selective serotonin reuptake inhibitor medications on baseline neurocognitive testing. Arch Clin Neuropsychol. 2022;37:633-40.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive and functional heterogeneity in depressed youth. Neuropsychopharmacology. 2021;46:783-90.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis and systematic review of Memory Specificity Training (MeST) in the treatment of emotional disorders. Behav Res Ther. 2019;116:36-51.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of cognitive impairment in patients with substance use disorder. Drug Alcohol Rev. 2019;38:435-42.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairments in early-detoxified alcohol-dependent inpatients and their associations with socio-demographic, clinical and psychological factors: An exploratory study. Neuropsychiatr Dis Treat. 2020;16:1705-16.
- [CrossRef] [PubMed] [Google Scholar]
- A dynamic method, analysis, and model of short-term memory for serial order with clinical applications. Psychiatry Res. 2020;294:113494.
- [CrossRef] [PubMed] [Google Scholar]
- Self-perception of mental illness, and subjective and objective cognitive functioning in people with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:967-76.
- [CrossRef] [PubMed] [Google Scholar]
- A cross-sectional study on associations between BDNF, CRP, IL-6 and clinical symptoms, cognitive and personal performance in patients with paranoid schizophrenia. Front Psychiatry. 2022;13:943869.
- [CrossRef] [PubMed] [Google Scholar]
- Modelling associations between neurocognition and functional course in young people with emerging mental disorders: A longitudinal cohort study. Transl Psychiatry. 2020;10:22.
- [CrossRef] [PubMed] [Google Scholar]
- Examining cognitive change in magnetic resonance-guided focused ultrasound capsulotomy for psychiatric illness. Transl Psychiatry. 2020;10:397.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive impairment in severe mental illness. Comparative study with Spanish speaking patients. Brain Sci. 2021;11:389.
- [CrossRef] [PubMed] [Google Scholar]
- Neural and functional correlates of impaired reading ability in schizophrenia. Sci Rep. 2019;9:16022.
- [CrossRef] [PubMed] [Google Scholar]
- Slow-wave sleep predicts long-term social functioning in severe mental illness. PLoS One. 2018;13:e0202198.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment in the cooccurrence of alcohol dependence and major depression: Neuropsychological assessment and event-related potentials analyses. Heliyon. 2022;8:e09899.
- [CrossRef] [PubMed] [Google Scholar]
- Six-month buprenorphine-naloxone treatment is associated with neurocognitive function improvement in opioid dependence. Indian J Psychiatry. 2022;64:199-208.
- [CrossRef] [PubMed] [Google Scholar]
- Memory changes following adjuvant temporo-parietal repetitive transcranial magnetic stimulation in schizophrenia. Indian J Psychiatry. 2021;63:66-9.
- [CrossRef] [PubMed] [Google Scholar]
- Implicit and explicit emotional memory recall in anxiety and depression: Role of basolateral amygdala and cortisol-norepinephrine interaction. Psychoneuroendocrinology. 2022;136:105598.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and cognitive significance of auditory sensory processing deficits in schizophrenia. Am J Psychiatry. 2018;175:275-83.
- [CrossRef] [PubMed] [Google Scholar]
- Cognition and daily life functioning among persons with serious mental illness: A cluster analytic examination of heterogeneity on the Test of Grocery Shopping Skills. Neuropsychology. 2021;35:57-68.
- [CrossRef] [PubMed] [Google Scholar]
- Applying speech technologies to assess verbal memory in patients with serious mental illness. NPJ Digit Med. 2020;3:33.
- [CrossRef] [PubMed] [Google Scholar]
- Somatosensory-motor dysconnectivity spans multiple transdiagnostic dimensions of psychopathology. Biol Psychiatry. 2019;86:779-91.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic overlap profiles of cognitive ability in psychotic and affective illnesses: A multisite study of multiplex pedigrees. Biol Psychiatry. 2021;90:373-84.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of cognitive remediation for schizophrenia: Efficacy and the role of participant and treatment factors. Schizophr Bull. 2021;47:997-1006.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive deficits in bipolar disorders: Implications for emotion. Clin Psychol Rev. 2018;59:126-36.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: A COSMIC collaboration cohort study. PLoS Med. 2019;16:e1002853.
- [Google Scholar]
- Longitudinal neurocognitive effects of combined electroconvulsive therapy (ECT) and pharmacotherapy in major depressive disorder in older adults: Phase 2 of the PRIDE study. Am J Geriatr Psychiatry. 2022;30:15-28.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of subjective cognitive deficits in patients with mild cognitive impairment. Psychogeriatrics. 2022;22:210-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychological predictors of performance-based measures of functional capacity and social skills in individuals with severe mental illness. J Psychiatr Res. 2018;102:201-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive functioning in ultra-high risk for psychosis individuals with and without depression: Secondary analysis of findings from the NEURAPRO randomized clinical trial. Schizophr Res. 2020;218:48-54.
- [CrossRef] [PubMed] [Google Scholar]
- Remediating reduced memory specificity in bipolar disorder: A case study using a Computerized Memory Specificity Training. Brain Behav. 2019;9:e01468.
- [CrossRef] [PubMed] [Google Scholar]
- Reaction time and visual memory in connection to alcohol use in persons with bipolar disorder. Brain Sci. 2021;11:1154.
- [CrossRef] [Google Scholar]
- The THINC-it tool for cognitive assessment and measurement in major depressive disorder: Sensitivity to change. Front Psychiatry. 2020;11:546.
- [CrossRef] [PubMed] [Google Scholar]
- Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry. 2018;75:270-9.
- [CrossRef] [PubMed] [Google Scholar]
- Active cigarette smoking is associated with increased age-related decline on measures of visuospatial learning and memory and executive function in alcohol use disorder. Alcohol Alcohol. 2022;57:656-63.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychological findings in recent onset schizophrenia and borderline personality disorder: A comparison study. Actas Esp Psiquiatr. 2019;47:7-15.
- [Google Scholar]
- Remote ecological momentary testing of learning and memory in adults with serious mental illness. Schizophr Bull. 2021;47:740-50.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of recombinant human erythropoietin on cognition and neural activity in remitted patients with mood disorders and first-degree relatives of patients with psychiatric disorders: a study protocol for a randomized controlled trial. Trials. 2018;19:611.
- [CrossRef] [PubMed] [Google Scholar]
- Outdoor air pollution exposure and inter-relation of global cognitive performance and emotional distress in older women. Environ Pollut. 2021;271:116282.
- [CrossRef] [PubMed] [Google Scholar]
- Music and neuro-cognitive deficits in depression. Front Psychol. 2022;13:959169.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between cytokines and verbal memory in individuals with schizophrenia and their unaffected siblings. Neuroimmunomodulation. 2018;25:334-9.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between residual depressive symptoms, cognition, and functioning in patients with euthymic bipolar disorder: Results from the FACE-BD cohort. Br J Psychiatry. 2017;211:381-7.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of remote administration of the MATRICS Consensus Cognitive Battery for individuals with severe mental illness. Schizophr Res Cogn. 2022;27:100226.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with poor functional outcome in bipolar disorder: Sociodemographic, clinical, and neurocognitive variables. Acta Psychiatr Scand. 2018;138:145-54.
- [CrossRef] [PubMed] [Google Scholar]
- Housing stability and neurocognitive functioning in homeless adults with mental illness: A subgroup analysis of the at home/Chez Soi study. Front Psychiatry. 2019;10:865.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal spontaneous gamma power is associated with verbal learning and memory dysfunction in schizophrenia. Front Psychiatry. 2020;11:832.
- [CrossRef] [PubMed] [Google Scholar]
- Divergence of subjective and performance-based cognitive gains following cognitive training in schizophrenia. Schizophr Res. 2019;210:215-20.
- [CrossRef] [PubMed] [Google Scholar]
- Compensatory cognitive training for people with severe mental illnesses in supported employment: A randomized controlled trial. Schizophr Res. 2019;203:41-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment in marginally housed youth: Prevalence and risk factors. Front Public Health. 2019;7:270.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in the cognitive function of first-diagnosed, drug-naïve depressed patients: An observational case-control study. Journal of affective disorders. 2020;276:461-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association between decreased serum TBIL concentration and immediate memory impairment in schizophrenia patients. Sci Rep. 2019;9:1622.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory cytokines, cognition, and response to antidepressant treatment in patients with major depressive disorder. Psychiatry Res. 2021;305:114202.
- [CrossRef] [PubMed] [Google Scholar]
- High-frequency cardiopulmonary coupling during sleep correlates with memory in depressed patients: A pilot study. J Affect Disord. 2020;270:118-23.
- [CrossRef] [PubMed] [Google Scholar]
- The interactive effects of stress and coping style on cognitive function in patients with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:523-30.
- [CrossRef] [PubMed] [Google Scholar]
- Neurobehavioral moderators of post-traumatic stress disorder (PTSD) trajectories: Study protocol of a prospective MRI study of recent trauma survivors. Eur J Psychotraumatol. 2019;10:1683941.
- [CrossRef] [PubMed] [Google Scholar]
- Personality in autism spectrum disorder: Associations with face memory deficit and theory of mind. Cogn Behav Neurol. 2021;34:117-28.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive predictors of treatment outcomes in cognitive processing therapy for post-traumatic stress disorder: Study protocol. Front Psychol. 2021;12:625669.
- [CrossRef] [PubMed] [Google Scholar]
- Executive dysfunction in eating disorders: Relationship with clinical features. Prog Neuropsychopharmacol Biol Psychiatry. 2023;120:110649.
- [CrossRef] [PubMed] [Google Scholar]
- Cognition in recent suicide attempts: Altered executive function. Front Psychiatry. 2021;12:701140.
- [CrossRef] [PubMed] [Google Scholar]
- A computerized neuropsychological evaluation of cognitive functions in a subclinical obsessive-compulsive sample. J Behav Ther Exp Psychiatry. 2018;59:142-9.
- [CrossRef] [PubMed] [Google Scholar]
- Posttraumatic stress disorder-associated cognitive deficits on the repeatable battery for the assessment of neuropsychological status in a veteran population. Fed Pract. 2021;38:28-34.
- [CrossRef] [PubMed] [Google Scholar]
- Association of genetic risk for rheumatoid arthritis with cognitive and psychiatric phenotypes across childhood and adolescence. JAMA Netw Open. 2019;2:e196118.
- [CrossRef] [PubMed] [Google Scholar]
- MoCA vs. MMSE of fibromyalgia patients: The possible role of dual-task tests in detecting cognitive impairment. J Clin Med. 2021;10:125.
- [CrossRef] [PubMed] [Google Scholar]
- Anxiety severity and cognitive function in primary care patients with anxiety disorder: A cross-sectional study. BMC Psychiatry. 2021;21:617.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired executive functioning of sexual assault survivors with acute stress disorder. J Clin Med. 2018;7:362.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot randomized controlled trial of goal management training in Canadian military members, veterans, and public safety personnel experiencing post-traumatic stress symptoms. Brain Sci. 2022;12:377.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive deficits in obsessive-compulsive disorder: A selective review. Indian J Psychiatry. 2019;61(Suppl 1):S30-6.
- [CrossRef] [PubMed] [Google Scholar]
- Discrepancies in Wechsler adult intelligent scale III profile in adult with and without attention-deficit hyperactivity disorder. Neuropsychopharmacol Rep. 2020;40:166-74.
- [CrossRef] [PubMed] [Google Scholar]
- Differences in cognitive profile of psychogenic nonepileptic and epileptic seizure patients. Indian J Private Psychiatry. 2020;14:63.
- [CrossRef] [Google Scholar]
- Attention impairment in motor functional neurological disorders: A neuropsychological study. J Neurol. 2022;269:5981-90.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-NMDA receptor encephalitis and overlapping demyelinating disorder in a 20-year old female with borderline personality disorder: Proposal of a diagnostic and therapeutic algorithm for autoimmune encephalitis in psychiatric patients “case report”. BMC Psychiatry. 2021;21:355.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of the severe cognitive impairment rating scale with the mini-mental state examination and delirium rating scale-revised-98 for delirium: A cross-sectional study. Psychosomatics. 2017;58:643-51.
- [CrossRef] [PubMed] [Google Scholar]
- Midlife Vascular factors and prevalence of mild cognitive impairment in late-life in Mexico. J Int Neuropsychol Soc. 2022;28:351-61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation and neuropsychological profiles of functional cognitive disorder patients with and without co-morbid depression. Cogn Neuropsychiatry. 2019;24:152-64.
- [CrossRef] [PubMed] [Google Scholar]
- A potential association between retinal changes, subjective memory impairment, and anxiety in older adults at risk for Alzheimer's disease: A 27-month pilot study. Front Aging Neurosci. 2019;11:288.
- [CrossRef] [PubMed] [Google Scholar]
- Subjective memory complaint as a useful tool for the early detection of Alzheimer's disease. Neuropsychiatr Dis Treat. 2018;14:2451-60.
- [CrossRef] [PubMed] [Google Scholar]
- Translation, adaptation and validation of the five-word test (Test Delle 5 Parole, T5P) in an Italian sample: A rapid screening for the assessment of memory impairment. Geriatrics (Basel, Switzerland). 2022;7:49.
- [CrossRef] [PubMed] [Google Scholar]
- Risks and Benefits of screening for dementia in primary care: The Indiana university cognitive health outcomes investigation of the comparative effectiveness of dementia screening (IU CHOICE)trial. J Am Geriatr Soc. 2020;68:535-43.
- [CrossRef] [PubMed] [Google Scholar]
- Stages of objective memory impairment predict Alzheimer's disease neuropathology: Comparison with the clinical dementia rating scale-sum of boxes. J Alzheimer's Dis. 2021;80:185-95.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of the montreal cognitive assessment (MoCA) index scores: A comparison with the cognitive domain scores of the seoul neuropsychological screening battery (SNSB) Dement Neurocogn Disord. 2021;20:28-37.
- [CrossRef] [PubMed] [Google Scholar]
- Qualitative comparison of semantic memory impairment in patients with amnestic mild cognitive impairment based on ß-amyloid status. J Clin Neurol (Seoul, Korea). 2019;15:27-37.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between subjective memory decline and depression symptom intensity in older people. Results of the second wave of cognition of older people, education, recreational activities, nutrition, comorbidities, and functional capacity studies (COPERNICUS) J Clin Med. 2021;10:1334.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive profiles in persons with depressive disorder and Alzheimer's disease. Brain Commun. 2020;2:fcaa206.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer's disease course. Alzheimers Res Ther. 2020;12:5.
- [CrossRef] [Google Scholar]
- Evaluating cognitive profiles of patients undergoing clinical amyloid-PET imaging. Brain Commun. 2021;3:fcab035.
- [CrossRef] [PubMed] [Google Scholar]
- Memory training for adults with probable mild cognitive impairment: A pilot study. Aging mental Health. 2019;23:1433-41.
- [CrossRef] [PubMed] [Google Scholar]
- Can subjective memory complaints identify Aß positive and Aß negative amnestic mild cognitive impairment patients? J Alzheimers Dis. 2019;70:1103-11.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between memory impairment and depressive symptoms can exacerbate anosognosia: A comparison of Alzheimers disease with mild cognitive impairment. Aging Ment Health. 2019;23:595-601.
- [CrossRef] [PubMed] [Google Scholar]
- Independent effects of white matter hyperintensities on cognitive, neuropsychiatric, and functional decline: A longitudinal investigation using the National Alzheimer's coordinating center uniform data set. Alzheimers Res Ther. 2019;11:64.
- [CrossRef] [PubMed] [Google Scholar]
- Management of cognitive decline in Alzheimer's disease using a non-pharmacological intervention program: A case report. Medicine. 2020;99:e20128.
- [CrossRef] [PubMed] [Google Scholar]
- Self-and informant-reported memory complaints: Frequency and severity in cognitively intact individuals and those with mild cognitive impairment and neurodegenerative dementias. J Alzheimers Dis. 2018;65:1011-27.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of quality of life among older people with mild cognitive impairment attending urban primary care clinics. Clin Gerontol. 2020;43:441-54.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial. Neurology. 2019;93:e1474-84.
- [CrossRef] [Google Scholar]
- Associations between intra-individual neurocognitive variability and prospective memory in veterans with mild traumatic brain injury history and posttraumatic stress disorder. Arch Clin Neuropsychol. 2022;37:1221-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of a cognitive training program in people with mild cognitive impairment: A study in urban community. Asian J Psychiatry. 2018;35:18-23.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain. 2020;143:2831-43.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of ICMR neurocognitive toolbox for dementia in the linguistically diverse context of India. Front Neurol. 2021;12:661269.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic precision in the detection of mild cognitive impairment: A comparison of two approaches. Am J Geriatr Psychiatry. 2022;30:54-64.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning with 18F-fluorodeoxyglucose-PET gives valid diagnoses for the uncertain cases in memory impairment of Alzheimer's disease. Front Aging Neurosci. 2021;13:764272.
- [CrossRef] [PubMed] [Google Scholar]
- A longitudinal comparison between depressed patients receiving electroconvulsive therapy and healthy controls on specific memory functions. Prim Care Companion CNS Disord. 2020;22:19m02547.
- [CrossRef] [PubMed] [Google Scholar]
- Electroconvulsive therapy cognitive assessment (ECCA) tool: A new instrument to monitor cognitive function in patients undergoing ECT. J Affect Disord. 2020;269:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term cognitive and psychological functioning in post-electroconvulsive therapy patients. J ECT. 2019;35:27-34.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive and neurophysiological recovery following electroconvulsive therapy: A study protocol. Front Psychiatry. 2018;9:171.
- [CrossRef] [PubMed] [Google Scholar]
- Response rate and subjective memory after electroconvulsive therapy in depressive disorders with psychiatric comorbidity. J Affect Disord. 2021;292:276-83.
- [CrossRef] [PubMed] [Google Scholar]
- Physician estimated vs. self-reported subjective memory in depressed patients treated with electroconvulsive therapy. Nordic J Psychiatry. 2020;74:359-65.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of pulse width on subjective memory impairment and remission rate 6 months after electroconvulsive therapy. J ECT. 2020;36:272-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improved safety of hybrid electroconvulsive therapy compared with standard electroconvulsive therapy in patients with major depressive disorder: A randomized, double-blind, parallel-group pilot trial. Front Psychiatry. 2022;13:896018.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophr Res. 2010;123:59-63.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychol Rev. 2018;28:509-33.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of depressive episodes on cognitive deficits in early bipolar disorder: Data from the Systematic Treatment Optimization Programme for Early Mania (STOP-EM) Br J Psychiatry. 2014;205:36-43.
- [CrossRef] [PubMed] [Google Scholar]
- The illiterate brain and the neuropsychological assessment: From the past knowledge to the future new instruments. Appl Neuropsychol Adult. 2018;25:174-87.
- [CrossRef] [PubMed] [Google Scholar]
- A new assessment for episodic memory. Episodic memory test and caregiver's episodic memory test. Neurologia (Barcelona, Spain). 2013;28:488-96.
- [CrossRef] [PubMed] [Google Scholar]
- A SEMantic and EPisodic Memory Test (SEMEP) developed within the embodied cognition framework: Application to normal aging, Alzheimer's disease and semantic dementia. Front Psychol. 2017;8:1493.
- [CrossRef] [PubMed] [Google Scholar]
- Battery for ECT related cognitive deficits (B4ECT-ReCoDe): Development and validation. Asian J Psychiatr. 2013;6:243-8.
- [CrossRef] [PubMed] [Google Scholar]
- Current limitations of neuropsychological tests and assessment procedures. Clin Neuropsychol. 2019;33:200-8.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of executive functioning in psychiatric disorders: Functional diagnosis as the ouverture of treatment. Clin Neuropsychiatry. 2007;4:111-6.
- [Google Scholar]







