Translate this page into:
Parvimonas micra-related spondylodiscitis: A case report and systematic literature review
*Corresponding author: Calogero Velluto, Department of Aging, Orthopaedic and Rheumatological Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy. calogerovelluto@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mazzella G, Velluto C, Caredda M, Messina F, Perna A, Meluzio M, et al. Parvimonas micra-related spondylodiscitis: A case report and systematic literature review. J Neurosci Rural Pract. 2024;15:415-24. doi: 10.25259/JNRP_72_2024
Abstract
The purpose of this study is to report a case of Parvimonas micra-related spondylodiscitis (PMSD) and conduct a systematic review of the literature to identify clinical, microbiologic, and radiographic assessment and treatment outcomes. This research was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A systematic review of the Literature indexed in PubMed, MEDLINE, and Scopus databases was performed from 1970 to December 2023 using search terms “vertebral,” “spinal,” “infection,” “spondylodiscitis,” “discitis,” “osteomyelitis,” “Parvimonas,” and “micra.” The systematic review identified 34 cases of PMSD in addition to the reported case after the screening of 472 titles and abstracts. Patients reported several clinical presentations, with back pain being the most common symptom. Treatment strategies included antibiotic therapy alone or in combination with surgery, resulting in favorable outcomes for the majority of patients. However, challenges such as delayed diagnosis and lack of standardized treatment protocols were observed. The P. micra is an emerging pathogen in spinal infections, particularly in patients with predisposing factors. This study highlights the need for heightened awareness among healthcare providers regarding the potential role of anaerobic bacteria in spondylodiscitis. Standardized diagnostic and treatment protocols are warranted to improve outcomes for patients with PMSD.
Keywords
Spondylodiscitis
Vertebral osteomyelitis
Parvimonas micra
Anaerobic bacteria
Spinal infections
INTRODUCTION
Pyogenic Spondylodiscitis is uncommon; however, it is associated with several pathological conditions and has a mortality of up to 20%.[1] This condition requires long-term antibiotic therapy and, in some cases, surgical treatment.[2-4] The pathophysiology of spondylodiscitis is linked to the introduction of pyogenic bacteria, either directly during spinal surgery or through the bloodstream, resulting in the infectious deterioration of the vertebral body and intervertebral disc.[5] The incidence of spondylodiscitis, particularly among individuals aged 75 years and older, has considerably increased.[6] The clinical presentation of pyogenic spondylodiscitis typically manifests as back pain or radiating discomfort, followed by fever, neurological deficits, and systemic symptoms such as weight loss and night sweats.[7] Elevation of laboratory parameters, including inflammatory markers such as C-reactive protein, is commonly observed at presentation and tends to correlate with the response to treatment.[8] A microbiological diagnosis of the responsible pathogen is crucial for effective treatment and is linked to improved clinical outcomes.[9] Despite its rarity, anaerobic spondylodiscitis, accounting for <3% of cases of pyogenic vertebral osteomyelitis, should always be considered and pursued through microbiological investigation.[5] Spondylodiscitis is often caused by Gram-positive aerobic bacteria, such as Staphylococcus aureus, with a reported incidence of around 80%.[10] Other pathogens, such as Escherichia coli (a Gram-negative aerobic bacteria) and Mycobacterium tuberculosis, are also reported in the Literature.[11] Anaerobic bacteria are rarely responsible for spondylodiscitis, include Parvimonas micra, a Gram-positive anaerobic coccus (GPAC), typically found in the oral cavity, respiratory system, gastrointestinal tract, and the female genitourinary tract. Originally identified as Peptostreptococcus micros, the organism was reclassified as P. micra in 2006.[12] It is widely recognized as a principal oral pathogen[13,14] and has also been implicated in meningitis,[15] cervical and cerebral abscesses,[16,17] infectious endocarditis,[18] and rarely spondylodiscitis.[19] The aim of this study is to report a case of P. micra-related spondylodiscitis (PMSD) and to conduct a systematic review of the literature to identify clinical, microbiologic, and radiographic assessments, offering insights into treatment options and clinical outcomes.
MATERIALS AND METHODS
This research was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Figure 1].[20]
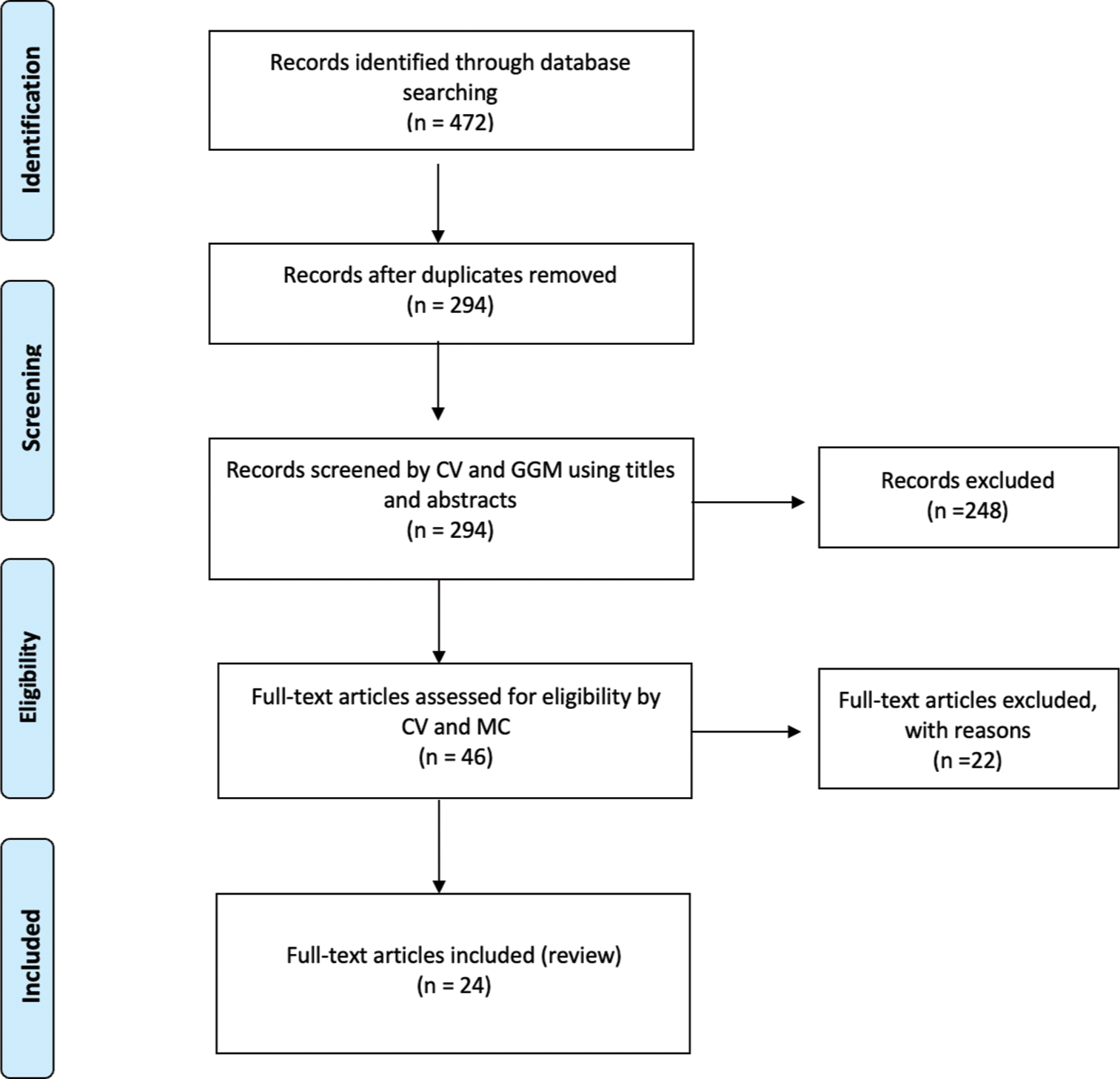
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow-chart. n: Number of records, GGM: Giovan Giuseppe Mazzella, CV: Calogero Velluto, MC: Matteo Caredda.
Search strategy
A systematic review of the Literature indexed in PubMed, MEDLINE, and Scopus databases, using search terms: “vertebral,” “spinal,” “infection,” “spondylodiscitis,” “discitis,” “osteomyelitis,” “parvimonas,” “micra,” and their medical subject headings (MeSH) terms in any possible combination, using the Boolean operator “AND” and “OR,” was performed from 1970 to December 2023. The search was reiterated until December 15, 2023. The reference lists of relevant studies were screened to identify other studies of interest.
Inclusion and exclusion criteria
Included in this review are studies reporting demographic features, symptoms, diagnostic settings, treatment, possible complications, and outcomes in patients with PMSD. Only articles written in English with available abstracts were included in the study. Excluded from this review were reports of surgical technique, expert opinions, animal studies, unpublished reports, cadaver or in vitro investigations, book chapters, and abstracts from scientific meetings.
Data collection
Two authors (G.G.M and C.V.) independently conducted the research by title and abstract. If the articles met the inclusion criteria, the full text was obtained and subsequently reviewed. Any discordance was resolved through consensus with a third author (M.C.). The reported items/data from the included articles encompassed: the age and gender of the patients, location of the PMSD, type of surgical treatment performed, underlying conditions, presence and location of any abscesses, presence of any immunosuppressive therapy, antibiotic therapy used, duration of antibiotic therapy, outcome, and complications.
Statistical analysis
Numbers software (Apple Inc., Cupertino, CA) was used to tabulate the obtained data. Categorical variables are presented as frequencies and percentages. Continuous variables are expressed as means and standard deviations.
CASE REPORT
In March 2021, a 64-year-old man with a history of Parkinson’s disease and diabetes, treated with L-Dopa, dopamine agonists, and oral hypoglycemic agents, was admitted to our emergency unit due to worsening back pain, associated with urinary incontinence and a walking deficit. He had undergone total hip arthroplasty and total knee arthroplasty in 2001 following multiple fractures from a car accident. A history of chronic low back pain led to an L3-L4 discectomy at another hospital in June 2019, which initially significantly alleviated his symptoms. However, 18 months post-surgery, his low back pain gradually returned and worsened. A magnetic resonance imaging (MRI) conducted 20 days before his current presentation revealed L2-L3-L4 spondylodiscitis with hyperintensity in T2 and STIR sequences [Figure 2].
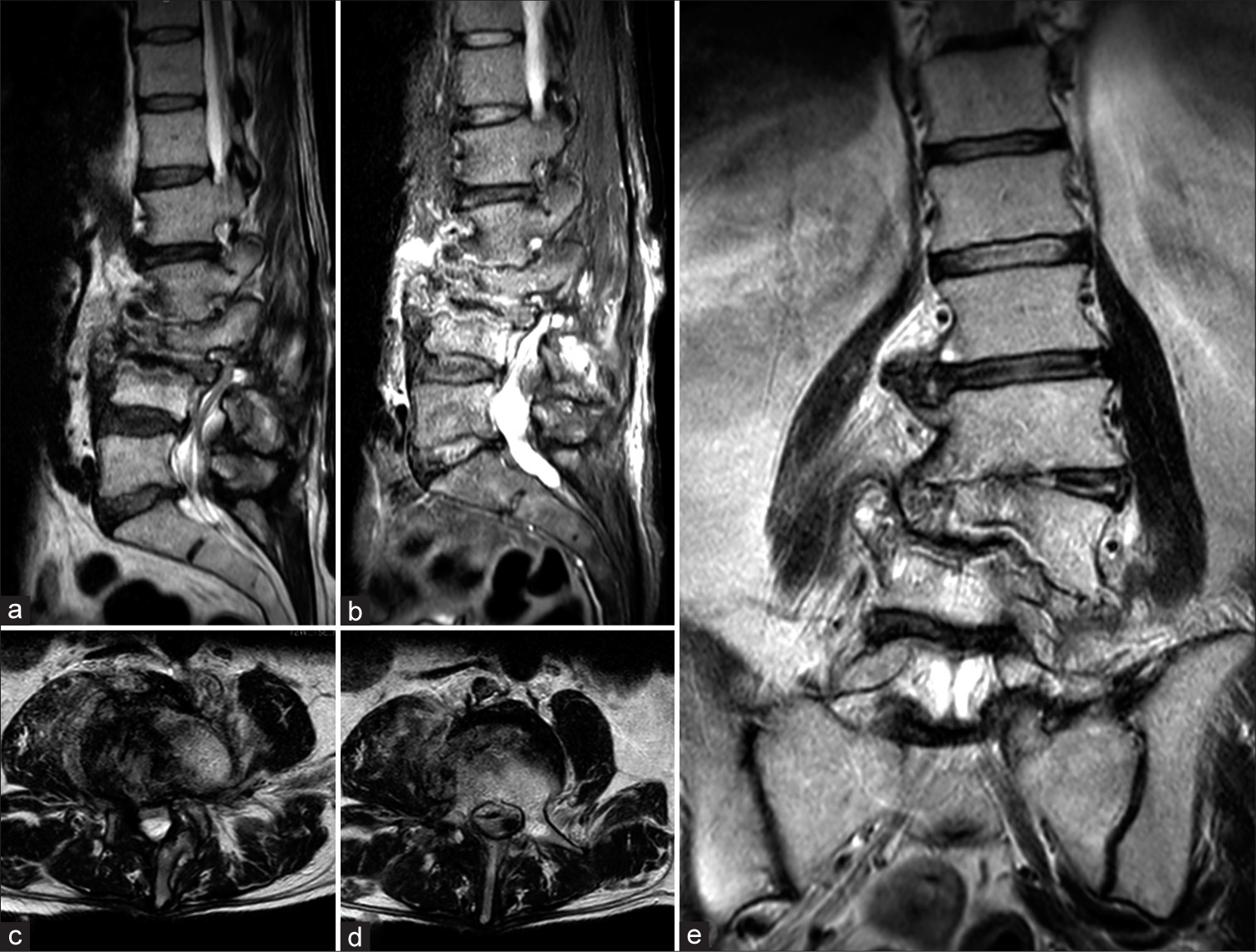
- (a) Sagittal T2 and (b) short T1 inversion recovery (STIR)-weighted images showing hyperintensity of L2, L3, and L4 with alteration of vertebral bodies. (c and d) Axial T2-weighted images showing hyperintensity of the right iliopsoas and of the vertebral body of L4. (e) Coronal T2-weighted view showing L3 collapse with complete disruption of L2-L3 and L3-L4 discs.
On arrival at the emergency room, a neurological examination and complete blood tests were performed. He showed no motor deficits in the upper limbs, but hypoesthesia and motor deficits were observed in the right lower limb (3/5 MRC in hip flexion, leg extension, ankle flexion, and extension). Patellar and Achilles reflexes were absent in the right limb. The visual analog scale for back pain was rated 8, and sensory disturbances in the saddle area and loss of visceral functions were noted. The latest MRI scans suggested cauda equina syndrome. Complete blood counts and blood chemistry tests were within normal limits for his age, except for elevated white blood cells at 22.96 × 109/L, neutrophils at 7.75 × 109/L, and mild thrombocythemia (578 × 109/L). Two sets of blood cultures were taken, indicating high inflammation rates with procalcitonin at 0.5 mg/dL and C-reactive protein at 98 mg/dL.
Due to worsening neurological symptoms, decompressive surgery was necessary. Under general anesthesia and in a prone position, a debulking of pseudomembranous tissue with a right L3 laminoarthrectomy was performed, although no purulent material was found. Large biopsy samples were taken for histological and microbiological evaluation. The intraoperative findings suggested a low likelihood of infection; hence, no empirical antibiotic therapy was initiated. The patient was discharged two days later in good clinical and neurological condition, free from back pain.
A post-operative computed tomography (CT) scan was conducted [Figure 3], and a lumbar custom-made brace was prescribed for early mobilization. Close outpatient follow-up was initiated to monitor clinical outcomes. Histological analysis confirmed chronic inflammation, and the intraoperative samples tested positive for P. micra. Consequently, targeted antibiotic therapy with amoxicillin/clavulanate (Augmentin 875/125 mg every 6 h) was initiated. One month post-procedure, neurological examinations showed no signs suggestive of cauda equina syndrome. The patient ceased antibiotic therapy two months later and began weaning from the brace. Conservative treatment continued for three months until a decrease in all inflammatory markers, including C-reactive protein, was observed. At the 12-month follow-up, he was in good health, without neurological signs or limitations in walking. A CT scan confirmed vertebral fusion between L2, L3, and L4 [Figure 4]. The patient’s postoperative follow-up was managed by a multidisciplinary team including a spine surgeon and an infectious disease specialist. Two years post-event, the patient reported no low back pain and an Oswestry Disability Index score of 22%. All procedures performed adhered to the 1964 Helsinki Declaration. Due to the retrospective nature of the research and the use of de-identified patient data, the Institutional Review Board waived the need for ethical review and approval. Written informed consent for scientific purposes and clinical data collection was obtained in accordance with institutional protocols.
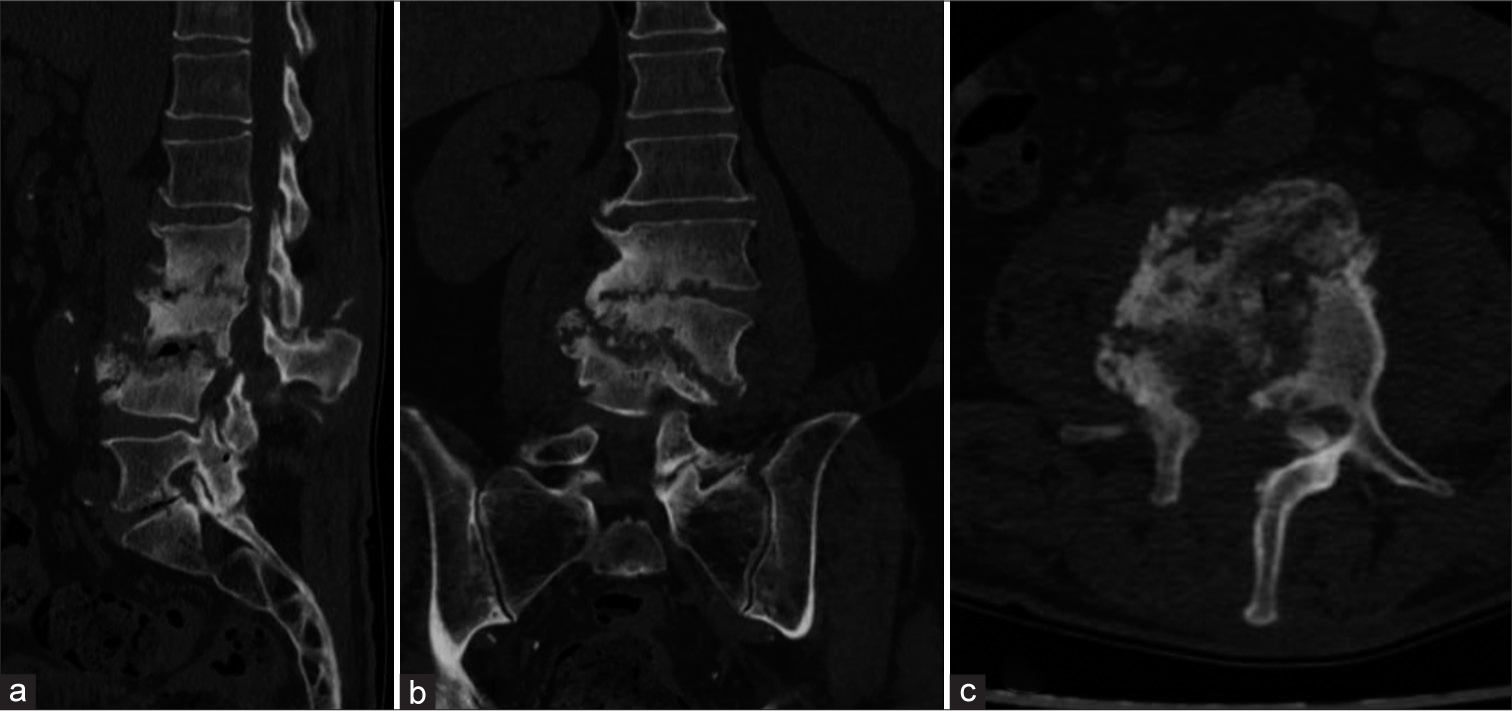
- (a) Sagittal, (b) coronal, and (c) axial post-operative computed tomography scans of the patient affected by Parvimonas micra-related spondylodiscitis, showing complete structural alteration of L2, L3, and L4 and the presence of laminectomy, as shown in the (c) axial view.
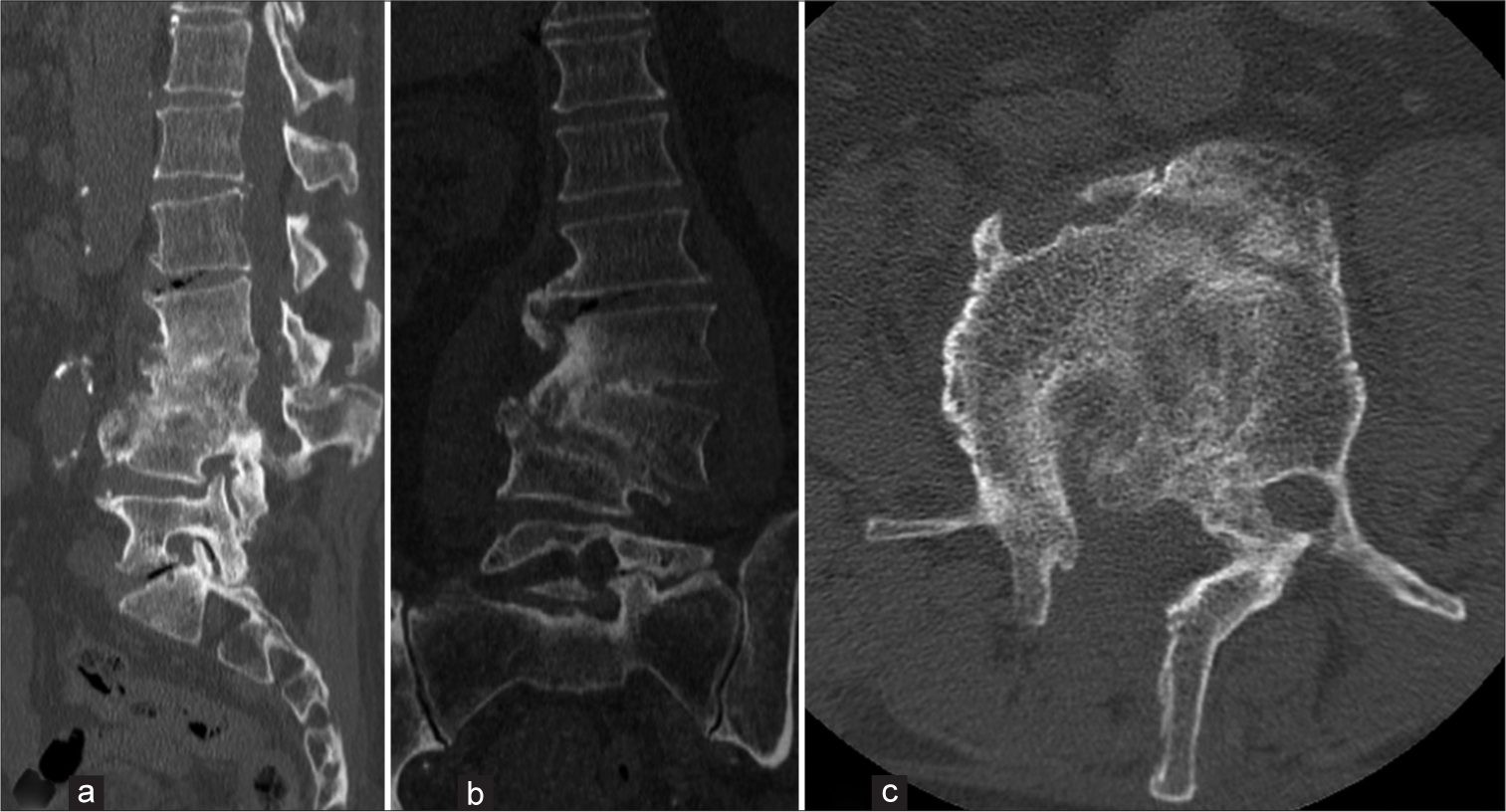
- (a) Sagittal, (b) coronal, and (c) axial computed tomography scan images at 12 months follow-up, showing vertebral fusion of L2-L3-L4.
LITERATURE REVIEW
After screening 472 titles and abstracts, we identified 46 papers deemed eligible for full-text analysis. Among these, 24 studies meeting inclusion criteria were ultimately included in the review. In total, including our report, 35 patients affected by PMSD were analyzed in this systematic review.[19,21-43]
Demographical data
The mean age of the included patients was 65.3 (8–86) years. Two (5.7%) were pediatric (<18 years old), and 22 (62.8%) were elderly (>65 years old). Among the included patients, 19 (54.3%) were male, and 16 (45.7%) were female. Demographic and clinical data are presented in Table 1.
| Author | Year | Age, years | Sex | Initial presentation | Comorbidities | Surgical treatment | Antibiotic treatment | Primary focus of infection | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mazzella et al. (current study) |
2024 | 64 | M | Lower back pain associated with urinary incontinence and walking deficit | Parkinson disease, diabetes, THA, TKA, L3-L4 discectomy | Debridement and L3 laminoarthrectomy and decompression | Amoxicillin/clavulanate 875/125 mg cpr OS every 6 h for 2 months | Unknown | No relapse |
| Kalmoukos et al.[21] | 2023 | 68 | F | Lower back pain and lower extremities pain | Rheumatoid arthritis, Osteoporotic vertebral fractures T12-L1 | None | Penicillin, clindamycin, 8 weeks | Hematogenous | No relapse |
| Paul et al.[22] | 2023 | 70 | F | Lower back pain | Chronic lymphocytic leukemia in remission, autoimmune hemolytic anemia, DM, hypertension, hypothyroidism, right THA | T9-L3 stabilization with T12 laminectomy | Vancomycin and cefepime 1 week; on 7th day meropenem 1 g 3 times daily, based on the positive Parvimonas micra report | Dental caries in the left maxillary molar | No relapse |
| Shimizu et al.[23] | 2022 | 54 | M | Persistent fever and pulsated headaches | Hypertension and stroke | None | NO (transient bacteremia that resolved spontaneously) | History of dental treatment, oral contamination, a painful mouth | No relapse |
| Durovic et al.[24] | 2022 | 82 | M | Acute immobilizing pain of the lower back and in both shoulder joints | Renal failure Gout Decompressive spinal surgery due to spinal stenosis in L1-L5 |
Acute paraplegia resulting from the abscesses, emergency decompression T12–L5 with laminectomy of L2-4 | Empiric antibiotic therapy with amoxicillin/clavulanic acid | Several tooth root granulomas | Death unrelated to spondylodiscitis |
| 69 | M | Lower back pain for 3 weeks and new hyposensitivity of the left thigh | Coronary heart disease Renal failure DM II Previous spinal surgery: decompression and left discectomy L2/3 |
CT-guided biopsy | 14 days of IV amoxicillin/clavulanic acid; oral amoxicillin for an additional 4 weeks | A single episode of fever following a tooth extraction 10 days before the back pain began | No relapse | ||
| 72 | M | Back pain for 2 weeks | Parkinson’s disease | CT-guided biopsy; followed by an open discectomy with drainage of the abscess | Empiric antibiotic therapy with amoxicillin/clavulanic acid; after identification penicillin IV for 2 weeks, and later amoxicillin orally for an additional 4 weeks | Dental procedure | Not reported | ||
| 72 | F | Persistent lower back pain for 6 weeks | Metastatic breast cancer with vertebral metastases | Fenestration and evacuation of the abscess | 5 weeks of IV treatment with amoxicillin/clavulanic acid; oral moxifloxacin for an additional 6 weeks | No primary focus of infection | Not reported | ||
| 72 | M | Lumbago for 3 months | Operated for spinal stenosis in L2–5 | Two CT-guided biopsies followed by transpedicular biopsy of L5 and the L4-5 disc | Penicillin and ertapenem IV for 2 weeks. Clindamycin for a further 12 weeks for treatment of chronic osteomyelitis | No primary focus of infection | No relapse | ||
| 63 | F | Lower back pain for 6 weeks | None | Transpedicular and disc open biopsy | Amoxicillin/clavulanic acid intravenously for 14 days, followed by oral amoxicillin for 6 weeks | Persistent inflammation of a molar following a dental procedure | Persistent pain | ||
| Miyazaki et al.[25] | 2019 | 83 | F | Lower back pain; tenderness and stabbing pain in his left thigh, accompanied by an erythematous rash | Prostatic cancer, DM, hypertension and hyperuricemia | None | 2 g of meropenem and 1200 mg of clindamycin daily | Not identified | Died of multiple organ failure on the 3rd day of his hospitalization |
| Yoo et al.[26] | 2019 | 77 | F | Acute on chronic lower back pain, aggravated by heavy lifting | Cerebrovascular accident, hypertension, hyperlipidemia and osteoporosis | Not required surgical intervention | IV ceftriaxone 2g once daily and IV metronidazole, dual cover with IV ceftriaxone and oral metronidazole (8-week course of ceftriaxone and 5-week course of metronidazole) | Not identified | Relapse-free 4 months after discharge |
| Van Duijvenbode et al.[27] | 2018 | 78 | M | Severe pain in right leg and in the lumbar spine | Bilateral THA, revision of the left THA, left TKA, hypertension, ulcerative colitis | Decompression of L2 and L3 and a posterior spondylodesis T12-L5 | Vancomycin and ciprofloxacin, after cultures results 12 g daily penicillin IV for 2 weeks, oral clindamycin 600 mg 3 times daily for 4 weeks | Laminectomy of L3-L5 performed 1.5 years before, collapse of the vertebral bodies of L2 and L3, compression of right nerve root and spinal stenosis at L3 | Occasional back pain at 1 year after surgery |
| Mizuta et al. | 2018 | 86 | F | Lower back pain | Not reported | None | Metronidazole 7 weeks | Unknown | Not reported |
| Cleaver et al.[28] | 2017 | 45 | F | 8 week history of lumbar back | Klippel–Trenaunay syndrome, copper intrauterine device in situ since 2009 | CT-guided vertebral biopsy; 6 days post-biopsy, percutaneous posterior spinal stabilization T10-L3 | Empirically postoperatively intravenous teicoplanin, piperacillin-tazobactam and amikacin; IV ertapenem, 1 g once daily, and oral clindamycin, 450mg 4 times daily, for a total of 6 weeks | Some years previously, dental cavity filling and some subsequent dental hygienist procedures, but good oral health | At 6 week follow-up, no spinal pain, with a tender but non-erythematous operative site |
| Higashi et al.[30] | 2017 | 67 | M | Progressive lower back pain for 2 months | DM II | Blood culture and transcutaneous vertebral biopsy; L4–S1 decompression and instrumented spinal fusion | IV ampicillin/sulbactam; on day 19 of admission, ampicillin (8 g/day) until day 72 of admission | Periodontitis | Recovered |
| Jones et al.[31] | 2015 | 72 | M | 4 weeks of back pain | None | Core biopsy | 6 weeks of IV piperacillin+tazobactam, then 2 weeks of oral amoxicillin+clavulanate | Uncomplicated tooth extraction 2 months before | Relapse-free after 12-month follow-up |
| 72 | F | 3 months of back pain, without fever or constitutional symptoms | Chronic osteoarthritis involving both hips and spine | Core biopsy of the T5 vertebral lesion and aspirate of the right paravertebral abscess | IV piperacillin+tazobactam for 4 weeks | Cervical spinal corticosteroid injections many years prior | Relapse-free after 5-month follow-up | ||
| George et al.[32] | 2015 | 49 | M | 3 week history of progressive low back pain | Spondylolisthesis with L3-L4 decompression and instrumented spinal fusion 6 months earlier | Debridement of soft tissue due to epidural abscess at L2-L3 and removal of the spinal hardware | IV ceftriaxone and oral metronidazole for 6 weeks | Dental work with tooth extraction | Normal inflammatory markers at 3 months follow-up |
| Gahier et al.[33] | 2015 | 59 | F | Cervical pain spreading to her left shoulder and asthenia of 6-week duration | None | None (6 blood cultures) | IV gentamicin, metronidazole and amoxicillin later replaced by amoxicillin alone for 14 weeks | Dental caries with an apical granuloma | Full recovery |
| 82 | F | Lower back pain | None | None (blood culture) | IV ceftriaxone and gentamicin replaced by amoxicillin for 6 weeks | Dental apical granuloma | Positive clinical outcome | ||
| 60 | F | Persistent lower back pain (2 months) despite a treatment with NSAIDs and morphine | None | None (blood culture) | IV ceftriaxone and gentamicin, later replaced by amoxicillin for 12 weeks | Portal of entry not identified | Positive clinical and biological outcome | ||
| Endo et al.[34] | 2015 | 55 | F | Lower back pain | None | Laminoplasty and debridement of the epidural abscess | High-dose IV sulbactam/ampicillin (6 g daily) for 6 weeks, followed by oral metronidazole (1500 mg daily) for 10 weeks in total |
Dental treatment before the onset of low back pain | Complete recovery |
| Medina et al.[35] | 2015 | 23 | F | 3-week history of fever associated with headache and dorsal paravertebral myalgia | None | None | Amoxicillin/clavulanic acid; rifampicin/clindamycin for 8 weeks |
Hematogenous | Successful |
| Pilmis et al.[36] | 2015 | 83 | M | Chronic lower back pain without fever | Left hip/right knee joint surgery and an ischaemic heart disease | Laminectomy L4-L5, four samples removed from the L4 and L5 vertebral bodies | IV amoxicillin and gentamicin for fifteen days followed by oral clindamycin and rifampicin for a total duration of 3 months |
No tooth infection or any portal of entry including gastrointestinal | No relapse was noted in a 6-months follow-up |
| Dahya et al.[37] | 2015 | 62 | M | 3-day history of fever, chills and intractable lumbar back pain | Hepatitis C, status after liver transplantation, Spinal stenosis Degenerative joints disease |
None | Vancomycin/Ceftriaxone | Endocarditis/Hematogenous | Successful |
| Gonzalez et al.[38] | 2014 | 62 | M | Lumbar back pain and weakness in lower left limb of 6 months duration | Hypertension DM Right hemiparesis Aphasia |
Percutaneous biopsy T7-T8 | Clindamycin for 4 months | Unknown | Successful |
| Uemura et al.[19] | 2014 | 83 | M | Lower back pain of 6-week duration, tenderness over L3 | Benign prostatic hypertrophy | Surgical biopsy of the vertebral bones | 3 g ampicillin-sulbactam every 6 h for 8 weeks, 625 mg oral amoxicillin-clavulanate every 8h for 4 weeks | Periodontitis | No relapse after 10 months |
| 85 | F | Worsening malaise, anorexia of 1-month duration | Hypertension | None, blood culture | Empiric doripenem IV for 12 days, IV ampicillin 3 g for 4 weeks, oral amoxicillin 500 mg every 6h for 8 weeks |
Periodontitis | No relapse | ||
| Fraisse et al.[39] | 2009 | 75 | M | Lower back pain | DM, hypercholesterolemia | None | Amoxicillin/Clavulanic acid+gentamicin, amoxicillin/clindamycin for 12 weeks | Hematogenous | Successful |
| Brook[40] | 2001 | 10 | M | 6 weeks of low back pain, fever, abdominal pain | None | None | Oral amoxicillin | Unknown | Successful |
| 8 | M | Low back pain, fever | None | None | Clindamycin | Unknown | Successful | ||
| Leder and Barlam[41] | 2000 | 70 | M | Lower back pain | Ulcerative colitis, osteoarthritis, benign prostatic hypertrophy | None | Amoxicillin/Metronidazole | Paraspinal abscess | Successful |
| Rousseau and Harlé[42] | 1998 | 82 | F | Not reported | None | None | Amoxicillin | Unknown | Successful |
| Papasian et al.[43] | 1986 | 70 | M | Not reported | Cataract operations, TURP, right inguinal herniorrhaphy | None | Nafcillin-clindamycin, 6 weeks | Unknown | Successful |
THA: Total hip arthroplasty, TKA: Total knee arthroplasty, DM: Diabetes mellitus, TURP: Transurethral resection of the prostate, IV: Intravenous, NSAIDs: Non-steroidal anti-inflammatory drugs.
Localization, presentation symptoms, and underlying conditions
In this literature review of PMSPD, several clinical scenarios are detailed, shedding light on the multifaceted nature of lower back pain associated with PMSD.
The PMSD involved the thoracic spine in three patients (8.6%) and the thoracolumbar spine area in seven patients (20%). The lumbar spine was involved in 20 (79.2%) patients, whereas the cervical spine was involved in two patients (5.7%, Gahier et al. and Medina et al.).[33,35] In one patient, no abnormalities were noted in the CT scan (Shimizu et al.).[23] A single level of spondylodiscitis was reported in 29 cases. On the other side, multi-level involvement was described in four patients. In 25.7% (9 patients), the infection was related to the presence of a paraspinal abscess that affected the psoas muscle. Among these, only one patient showed an intraspinal abscess (Durovic et al.).[24] In one patient, there was extraspinal localization: Miyazaki et al. reported the clinical history of a patient, who exhibited a significant pleural effusion in his left thoracic cavity, necessitating drainage through a thoracotomy tube.[25] Two patients experienced bloodstream infection with severe compromise of organs functionality. The most frequent presentation symptom was low back pain. Notably, the cases by Paul et al. (2023) and Yoo et al. (2019) reported acute exacerbations of lower back pain in individuals with chronic health conditions.[22,26] The study by Shimizu et al. (2022) described a unique case of persistent fever and pulsated headaches in a patient with a history of dental treatment, emphasizing the potential oral origin of PMSD.[23] Medina et al. reported a case of an atypical presentation of Lemierre’s syndrome.[35] Van Duijvenbode’s et al. case (2018) reported a patient with severe leg and lumbar spine pain postoperatively: laminectomy of L3-L5 was performed 1.5 years before his visit, due to lumbar stenosis as a result of degenerative lumbar deformity.[27] Cleaver et al. (2017) reported a case of PMSD related to KlippelTrenaunay syndrome.[29] Twelve patients (34.3%) had no comorbidities at the moment of hospitalization. No patients were immunocompromised, even though Miyazaki et al. reported a history of a patient with a background of prostatic cancer and diabetes mellitus (DM).[25] These conditions might have compromised his immune system and disrupted host defenses, potentially facilitating the entry of P. micra into his bloodstream. The most commonly reported predisposing conditions for PMSPD were previous spine or dental surgeries, although a primary focus of infection was frequently undetermined. In this review, 17 patients (48.6%) had no recorded details regarding the portal of infection. Uemura et al. suggested that advanced age might constitute another significant risk factor for P. micra-induced spondylodiscitis.[19]
Diagnosis
The diagnosis of PMSD varied among the included studies, with six authors, namely, Paul et al., Cleaver et al., Higashi et al., Jones et al., Pilmis et al., George et al., and Medina et al. relying on matrix-assisted laser desorption ionization time-of-flight mass spectrometry for a total of 8 patients (22.8%).[22,29-32,35,36] Shimizu et al., Durovic et al., Gahier et al., and Yoo et al. employed blood cultures for diagnosis, representing 11 patients (31.4%).[23,24,26,33] Van Duijvenbode et al. opted for intraoperative cultures.[27] Miyazaki et al., Endo et al., and Uemura et al. utilized 16S rRNA gene sequencing for three patients (8.6%).[19,25,34] Notably, Uemura et al. additionally utilized isolation by culture (RapID-ANA) in triplicate for one patient (2.85%).[19] However, Mizuta et al., Dahya et al., Gonzalez et al., Fraisse et al., Brook et al., Leder et al., Rousseau et al., and Papasian et al. did not disclose their specific diagnostic approaches in their respective studies.[28,37-43] This diversity underscores the need for standardized diagnostic protocols in the field of PMSD.
Treatment options
Antibiotic therapy alone was administered in 16 patients (45.7%). In 18 patients (51.4%), a combination of medical and surgical treatment was described, while one patient (2.8%) suffered a transient bacteremia resolved spontaneously. In two cases (5.7%), a CT-guided biopsy was performed, in one case a percutaneous biopsy (2.85%, Gonzalez et al.), in four cases (11.4%) a surgical biopsy was reported, in three cases (8.6%) abscess drainage, in four cases (11.4%) posterior decompression, and in five cases (14.3%) posterior decompression and fusion.[38] Combination antibiotic treatment was prescribed in all cases, except one. The most commonly used antibiotic was amoxicillin/clavulanic acid (40%), followed by clindamycin (31.4%) and gentamicin (14.3). In 31 (88.6%) cases, the duration of antibiotic therapy was reported. The mean duration was 61.2 days. Dahya et al., Brook and Rousseau et al. had not reported the duration of therapy.[37,40,42] At the time, the case report was published, antibiotic therapy was still ongoing in no case.
Outcomes
The clinical outcomes were reported in 33 cases (94.3%). Twenty-eight (80%) had a complete recovery: They were relapse free at the last follow-up visit. Three patients (8.6%) complained of persistent lumbar back pain but an improvement in the radiological picture was observed. Two patients (5.7%) died during follow-up from causes unrelated to spondylodiscitis. A complication was reported in one patient: A Clostridium difficile infection occurred three weeks after completion of antimicrobial therapy.[26]
DISCUSSION
The P. micra, a GPAC, typically inhabits the normal oropharyngeal flora. Formerly classified as Peptostreptococcus micros, this bacterium also resides as a commensal in the gastrointestinal and urogenital tracts, though it infrequently leads to serious infections.[12] Infections involving P. micra typically arise within polymicrobial infections, originating from established microbial colonies, and its dissemination through the bloodstream can result in metastatic infections. The literature most commonly documents cases of endodontic infections and oropharyngeal abscesses, with pleural empyema and intra-abdominal abscesses being less common.[13] Papasian et al. reported the first case of vertebral osteomyelitis secondary to P. micra in 1986.[43] Since then, only a few works on this topic can be found in the literature, indicating that PMSDs are a clinical entity still being defined.[27] Most cases of spondylodiscitis result from the blood-borne spread of bacteria originating from remote infection sites, predominantly involving S. aureus, and to a smaller degree, Enterobacteriaceae and Streptococci.[24] Recognizing the causative microorganism of spinal infection is essential for timely and effective management but can be challenging in some cases. Due to the poor sensitivity of blood cultures, specific growth needs and slow propagation of the bacterium, along with negative spinal biopsies, identifying the responsible pathogen often requires several attempts, which can delay treatment.[24] Typically, detecting anaerobic bacteria in spinal biopsies is uncommon, with Cutibacterium acnes and Bacteroides fragilis being the species most frequently found.[44] The elderly population is at the greatest risk of developing spinal infections, and the lumbar area is the most affected. Additional significant risk factors include DM, intravenous drug use, and previous spinal surgeries[11] and recent dental procedures. In a recent systematic review of the literature, Van Duijvenbode et al. suspected an odontogenic focus in 50% of cases;[27] moreover, Durovic et al., in a case series of six patients with PMSD, suspected a dental focus as the source of infection in four out of six patients, with a short rest between dental infection and symptoms of spondylodiscitis, indicating a clear correlation between recent dental procedures and the development of PMSD.[24] The clinical presentation of patients with PMSD is generally a history of worsening back pain, associated or not with mild systemic inflammatory signs. In cases where symptoms are more severe, it is common to administer empiric broad-spectrum antibiotics before pinpointing the infection’s source and identifying the responsible pathogen. According to the 2015 guidelines from the Infectious Diseases Society of America (IDSA), a 6-week course of parenteral antimicrobial treatment is advised for the majority of patients suffering from bacterial vertebral osteomyelitis.[45] In cases of acute neurological compromise or pyogenic abscesses requiring evacuation and drainage, surgical treatment must be associated in addition to targeted antibiotic therapy.[26] MRI of the spine is considered the decisive method for assessing patients exhibiting signs or symptoms that may indicate spondylodiscitis. If results are uncertain, CT-guided biopsies, or open biopsies when necessary, are regarded as the definitive diagnostic tools. The guidelines from the IDSA state that a positive blood culture for S. aureus is adequate to confirm the infection source when spondylodiscitis is identified through MRI.[45] We performed a systematic review of the literature to define a correct diagnostic approach to PMSD that allows for timely treatment, whether pharmacological or surgical, which would improve the prognosis of these patients. The systematic review revealed the increasing incidence of spondylodiscitis, particularly among individuals aged 75 years and older, and emphasizes the importance of timely diagnosis and effective treatment to improve clinical outcomes. The clinical presentation of pyogenic spondylodiscitis typically manifests as back pain or radiating discomfort, along with fever, neurological deficits, and systemic symptoms. Laboratory parameters such as inflammatory markers play a crucial role in diagnosis and treatment response monitoring. Furthermore, while Gram-positive aerobic bacteria, particularly S. aureus, are commonly implicated in spondylodiscitis, our study highlights the rare occurrence of anaerobic spondylodiscitis, with P. micra being identified as one such pathogen. Radiological outcomes in our case report play a pivotal role in the comprehensive evaluation of PMSD, providing crucial insights into disease progression and treatment response. As shown in Figure 2, there was a complete disruption of bone associated with degenerative changes, such as vertebral endplate erosion and disc space narrowing. These findings offer valuable diagnostic clues, while assessments of sagittal and coronal balance highlight mechanical instability and potential neurological compromise. In our case, the patient reported symptoms including radiculopathy and severe back pain, which prompted emergency decompression surgery. Furthermore, in accordance with infectious disease recommendations, antibiotic therapy was initiated along with immobilization using a brace. These radiological and clinical parameters contribute to a holistic approach to managing this challenging condition, guiding therapeutic decisions, and optimizing patient outcomes.
In addressing the distinctive characteristics and clinical considerations of PMSD, our analysis reveals several points of interest that warrant further elucidation in the results section of the manuscript. First, the impact of P. micra on spondylodiscitis appears to diverge from more common pathogens due to its anaerobic nature, potentially contributing to a delayed diagnosis and subsequent treatment initiation, as the organism requires specific conditions for growth and identification in cultures.[19,24,43] Radiologically, PMSD does not present with uniquely distinguishing features when compared to other causes of spondylodiscitis; however, the propensity for abscess formation, particularly paraspinal, may be more pronounced given the anaerobic and polymicrobial tendencies of the infection.[19,26,27] The treatment duration for PMSD, while not inherently longer, must be prudently tailored to each case, often employing a combination of surgical intervention and targeted antibiotic therapy, with the duration guided by the patient’s clinical response and the resolution of symptoms and laboratory markers of infection.[45] P. micra’s role in spondylodiscitis is further underscored by its association with dental procedures, a potential primary source for infection spread through hematogenous routes.[19,24] In addition, risk factors such as prior spinal surgery, immunocompromised states, and the presence of other comorbid conditions play a significant role in the susceptibility to PMSD, delineating the need for heightened vigilance and targeted diagnostic strategies in at-risk patient populations.[11,19,37] Collectively, these insights into the pathogenicity, diagnosis, and management of PMSD contribute to a better understanding of this unique clinical entity, emphasizing the need for prompt and appropriate diagnostic and therapeutic measures to mitigate its impact on patient outcomes.
Thus, a comprehensive evaluation encompassing both radiological outcomes and reported symptoms is essential in the management of PMSD, facilitating timely diagnosis, treatment planning, and monitoring of disease progression. Future research efforts should focus on elucidating the optimal diagnostic modalities, treatment strategies, and outcomes associated with PMSD. Collaborative endeavors are essential to accumulate larger datasets that can inform evidence-based guidelines for the management of this rare but potentially debilitating condition. Ultimately, our study contributes valuable insights to the existing literature and underscores the need for continued vigilance and research in the field of infectious spondylodiscitis, with specific attention to the role of P. micra as a causative pathogen.
CONCLUSION
P. micra is a potential causative agent in spinal infections, particularly in patients with underlying risk factors such as dental or gastrointestinal pathologies. The systematic literature review revealed a limited but growing body of evidence implicating P. micra in spondylodiscitis, emphasizing the need for heightened awareness among spine surgeons. Early detection is crucial for implementing timely and effective therapeutic interventions. In addition, the review sheds light on the diverse treatment approaches reported in the literature, emphasizing the absence of standardized guidelines for managing PMSD.
Acknowledgments
Giovan Giuseppe Mazzella and Calogero Velluto contributed equally.
Authors’ contributions
Conceptualization, GGM, CV, LP. Methodology, GGM, LP. Data curation, GGM, CV. Formal analysis, MCM, CV. Investigation, GGM, CV, FM, MC. Validation, CV, FM, MC, LP. Writing – original draft preparation, GGM, CV. Writing – review and editing GGM, CV. Visualization, FM, MC, LP. Supervision, LP. All authors have read and agreed to the published version of the manuscript.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: A systematic literature review. Eur Spine J. 2016;25:983-99.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation and outcomes after medical and surgical treatment versus medical treatment alone of spontaneous infectious spondylodiscitis: A systematic literature review and meta-analysis. Glob Spine J. 2018;8:49S-58.
- [CrossRef] [PubMed] [Google Scholar]
- Appropriate duration of post-surgical intravenous antibiotic therapy for pyogenic spondylodiscitis. BMC Infect Dis. 2018;18:468.
- [CrossRef] [PubMed] [Google Scholar]
- Skipped vertebral spontaneous spondylodiscitis caused by Granulicatella adiacens: Case report and a systematic literature review. J Clin Orthop Trauma. 2020;11:937-41.
- [CrossRef] [PubMed] [Google Scholar]
- Anaerobic spondylodiscitis: A retrospective analysis. BMC Musculoskelet Disord. 2022;23:788.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing incidence of spondylodiscitis in England: An analysis of the national health service (NHS) hospital episode statistics from 2012 to 2021. Brain Spine. 2023;3:101733.
- [CrossRef] [PubMed] [Google Scholar]
- Non tuberculous mycobacteria related spondylodiscitis: A case report and systematic literature review. Infez Med. 2020;28:425-35.
- [Google Scholar]
- Could serum procalcitonin play a role in an emergency setting for patients with pyogenic spondylodiscitis? Eur Rev Med Pharmacol Sci. 2022;26(1 Suppl):66-77.
- [Google Scholar]
- Epidemiology, microbiological diagnosis, and clinical outcomes in pyogenic vertebral osteomyelitis: A 10-year retrospective cohort study. Open Forum Infect Dis. 2018;5:ofy037.
- [CrossRef] [PubMed] [Google Scholar]
- Spondylodiscitis: Diagnosis and treatment options. Dtsch Arztebl Int. 2017;114:875-82.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal infection: State of the art and management algorithm. Eur Spine J. 2013;22:2787-99.
- [CrossRef] [PubMed] [Google Scholar]
- Proposal of Parvimonas gen. nov and Quatrionicoccus gen. Nov. as replacements for the illegitimate, prokaryotic, generic names Micromonas Murdoch and Shah 2000 and Quadricoccus Maszenan et al. 2002, respectively. Int J Syst Evol Microbiol. 2006;56:2711-3.
- [CrossRef] [PubMed] [Google Scholar]
- Gram-positive anaerobic cocci. Clin Microbiol Rev. 1998;11:81-120.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of subgingival periodontal pathogens--comparison of two sampling strategies. Clin Oral Investig. 2016;20:571-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteremic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe. 2015;34:161-3.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical spinal epidural abscess and meningitis due to Prevotella oris and Peptostreptococcus micros after retropharyngeal surgery. Intensive Care Med. 2004;30:1695.
- [CrossRef] [PubMed] [Google Scholar]
- Oesophageal pleural fistula presenting with Parvimonas micra infection causing cervical and brain abscesses. Anaerobe. 2017;47:233-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Peptostreptococcus micros endocarditis with teicoplanin. Clin Infect Dis. 1995;21:446-7.
- [CrossRef] [PubMed] [Google Scholar]
- Parvimonas micra as a causative organism of spondylodiscitis: A report of two cases and a literature review. Int J Infect Dis. 2014;23:53-5.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- [CrossRef] [PubMed] [Google Scholar]
- Anaerobic spondylodiscitis caused by Parvimonas micra in a rheumatoid arthritis patient: Case report and review of the literature. Mediterr J Rheumatol. 2023;34:525-30.
- [CrossRef] [PubMed] [Google Scholar]
- A rare anaerobic cause of vertebral osteomyelitis and psoas abscess: A case study. IDCases. 2023;34:e01900.
- [CrossRef] [PubMed] [Google Scholar]
- Infection route of Parvimonas micra: A case report and systematic review. Healthcare (Basel). 2022;10:1727.
- [CrossRef] [PubMed] [Google Scholar]
- Parvimonas micra as a rare cause of spondylodiscitis-case series from a single centre. Swiss Med Wkly. 2020;150:w20272.
- [CrossRef] [PubMed] [Google Scholar]
- Severe sepsis caused by Parvimonas micra identified using 16S ribosomal RNA gene sequencing following patient death. IDCases. 2019;19:e00687.
- [CrossRef] [PubMed] [Google Scholar]
- Parvimonas micra spondylodiscitis with psoas abscess. BMJ Case Rep. 2019;12:e232040.
- [CrossRef] [PubMed] [Google Scholar]
- Parvimonas micra spondylodiscitis: A case report and systematic review of the literature. J Orthop Case Rep. 2018;8:67-71.
- [Google Scholar]
- Metronidazole-induced encephalopathy in a patient with pyogenic spondylitis: A case report. BMC Musculoskelet Disord. 2018;19:336.
- [CrossRef] [PubMed] [Google Scholar]
- A case of polymicrobial anaerobic spondylodiscitis due to Parvimonas micra and Fusobacterium nucleatum. JMM Case Rep. 2017;4:e005092.
- [CrossRef] [PubMed] [Google Scholar]
- Spondylodiscitis due to Parvimonas micra diagnosed by the melting temperature mapping method: A case report. BMC Infect Dis. 2017;17:584.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of spondylodiscitis caused by Parvimonas micra. Intern Med J. 2015;45:1090-1.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed infection with Parvimonas micra following spinal instrumentation. Anaerobe. 2015;35(Pt B):102-4.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal infections caused by Parvimonas micra. Med Mal Infect. 2015;45:397-8.
- [CrossRef] [PubMed] [Google Scholar]
- First confirmed case of spondylodiscitis with epidural abscess caused by Parvimonas micra. J Infect Chemother. 2015;21:828-30.
- [CrossRef] [PubMed] [Google Scholar]
- Lemierre's syndrome: An unusual presentation. Med Mal Infect. 2015;45:328-30.
- [CrossRef] [PubMed] [Google Scholar]
- Spondylodiscitis due to anaerobic bacteria about a case of Parvimonas micra infection. Anaerobe. 2015;34:156-7.
- [CrossRef] [PubMed] [Google Scholar]
- Peptostreptococcus endocarditis presenting as lumbar discitis in an immunocompromised patient. Am J Med Sci. 2015;349:187-8.
- [CrossRef] [PubMed] [Google Scholar]
- Multifocal hematogenous vertebral osteomyelitis due to Parvimonas micra and a subsequent pleural effusion in a diabetic patient. Reumatol Clin. 2014;10:191-2.
- [CrossRef] [Google Scholar]
- Spondylodiscitis due to Peptostreptococcus spp: A case report. Joint Bone Spine. 2009;76:104-5.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of diskitis attributable to anaerobic bacteria in children. Pediatrics. 2001;107:26.
- [CrossRef] [PubMed] [Google Scholar]
- A case of paraspinal abscess and diskitis due to Peptostreptococcus micros. Clin Infect Dis. 2000;30:622-3.
- [CrossRef] [PubMed] [Google Scholar]
- Spondylitis caused by Peptostreptococcus. Clin Rheumatol. 1998;17:538-9.
- [CrossRef] [PubMed] [Google Scholar]
- Peptostreptococcal vertebral osteomyelitis. J Clin Microbiol. 1986;24:633-5.
- [CrossRef] [PubMed] [Google Scholar]
- Spondylodiscitis: Update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11-24.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26-46.
- [CrossRef] [PubMed] [Google Scholar]







