Translate this page into:
Advanced Magnetic Resonance Imaging of Glioblastoma Multiforme
Address for correspondence: Dr. Reddy Ravikanth, St. John's Medical College, Bengaluru - 560 034, Karnataka, India. E-mail: ravikanthreddy06@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 68-year-old male presented to the emergency department with a headache. He had no known comorbidities. His general physical and neurological examination was normal. He underwent an emergency magnetic resonance (MR) screening which showed a T1 heterogeneously hypointense, T2 heterogeneously hyperintense [Figure 1] lesion involving the subcortical and deep white matter of the right parietooccipital region. The lesion was noted to infiltrate across the midline through the splenium of corpus callosum. Perilesional edema was noted causing regional sulcal effacement. A focal area of diffusion restriction was noted in the inferomedial aspect of the lesion suggestive of high cellularity zone [Figure 2]. Tiny intralesional foci of blooming were noted suggestive of microbleeds [Figure 3]. Postcontrast T1-weighted fat saturated images showed evidence of peripheral heterogeneous enhancement [Figure 4]. Single-voxel MR spectroscopy (MRS) showed elevated choline, lactate, lipid peaks, and decreased N-acetylaspartate (NAA) peak in the intralesional [Figure 5] and perilesional [Figure 6] regions of the tumor. In addition, choline: NAA ratio was 1.7, choline: Creatinine ratio was 2.4, and NAA: Creatinine ratio was 1.41 in the intralesional voxel and choline:NAA ratio was 2.29, choline:Creatinine ratio was 1.36, and NAA:Creatinine ratio was 1.68 in the perilesional voxel. In assumption of a malignant tumor, we performed a stereotactic-guided right parietooccipital craniotomy with radical subtotal resection of the lesion, and the histopathological examination confirmed the presence of glioblastoma multiforme (GBM). The patient underwent additional radiotherapy and chemotherapy with concomitant temozolomide. Temozolomide dosed at 75 mg/m2/day, started the night before the first radiation treatment and given for 42 consecutive days. Dexamethasone with a dosage of 16 mg daily, administered in four equal doses was given to the patient postsurgery followed by a maintenance dose of 0.5–1 mg dose of dexamethasone daily. Postoperative MR imaging (MRI) revealed postoperative changes with no evidence of abnormal enhancement/residual tumor.
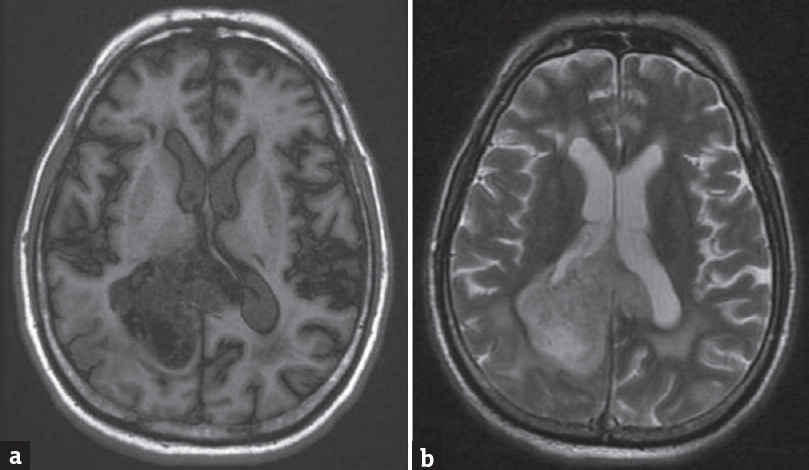
- (a) Axial T1-weighted magnetic resonance image showing a heterogeneously hypointense lesion involving the right parietooccipital white matter. (b) Axial T2-weighted magnetic resonance image showing a heterogeneously hyperintense lesion involving the right parietooccipital white matter with perilesional edema
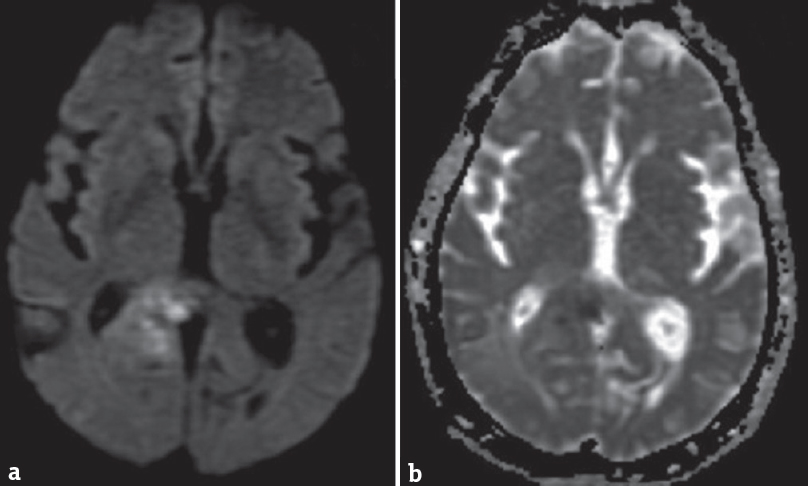
- (a) Axial diffusion-weighted magnetic resonance image and (b) apparent diffusion coefficient map showing focus of restriction within the lesion posterolateral to the splenium
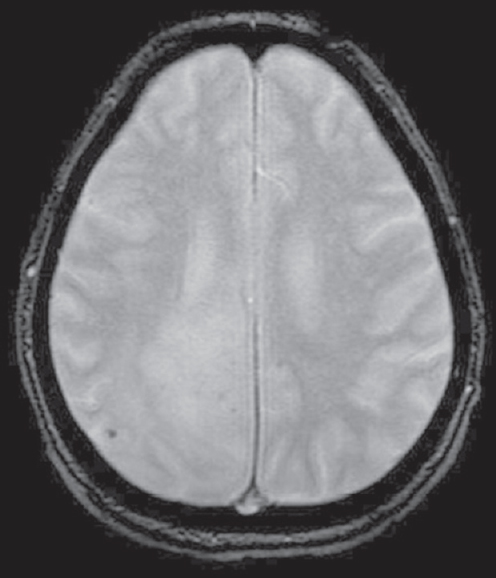
- T2-weighted gradient recalled echo image showing intralesional foci of blooming
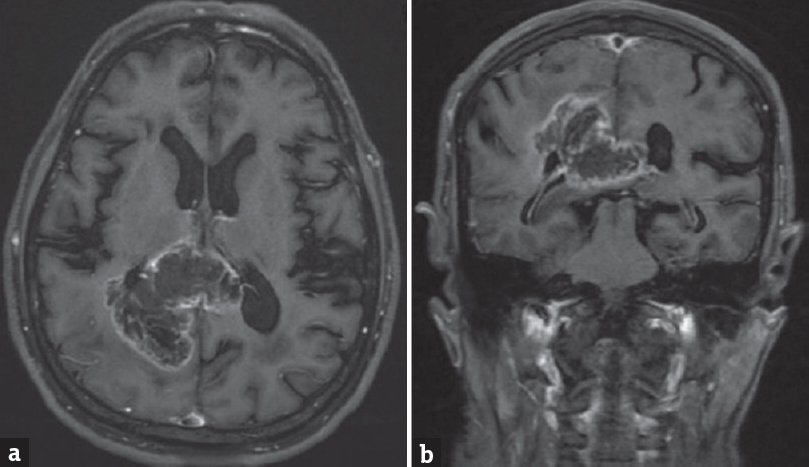
- T1-weighted postcontrast fat saturated images (a) axial sections and (b) coronal sections showing peripheral heterogeneous enhancement with infiltration across the midline through the splenium of the corpus callosum
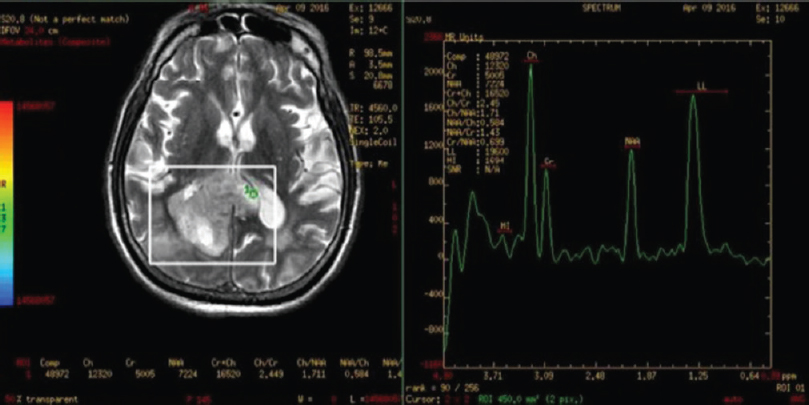
- Intralesional single voxel magnetic resonance spectroscopy images showing elevated choline, lactate, lipid peaks, and decreased N-acetylaspartate peak with elevated choline:N-acetylaspartate and choline:creatinine ratios and decreased N-acetylaspartate:creatinine ratio

- Perilesional single voxel magnetic resonance spectroscopy images, showing elevated choline, lactate, lipid peaks, and decreased N-acetylaspartate peak with elevated choline: N-acetylaspartate and choline: Creatinine ratios and decreased N-acetylaspartate: Creatinine ratio
DISCUSSION
GBM (WHO grade IV astrocytoma) is the most common primary brain malignancy, accounting for up to 15% of all intracranial neoplasms. It occurs most commonly in the middle age population (mean age of 55 years) and is frequently supratentorial in location’[1] An existing astrocytoma may dedifferentiate into a high-grade glioma, in which case they are secondary glioblastomas. GBMs usually form in the cerebral white matter, grow quickly, and can become very large before producing symptoms. Less than 10% form more slowly following degeneration of low-grade astrocytoma or anaplastic astrocytoma. These are called secondary GBMs and are more common in younger patients (mean age 45 vs. 62 years).[2] They are high-grade tumors that have classical MRI features as described in our patient. In MRS, elevated choline and reduced NAA suggest tumor turnover and neuronal loss; metabolite ratios (choline-creatinine, choline-NAA, NAA-creatinine, myoinositol-creatinine) correlate with tumor grade.[3]
CONCLUSION
GBM is the most common primary brain tumor, accounting for up to 15% of all intracranial neoplasms. MRI is the imaging modality of choice in the evaluation of gliomas, and can accurately detect high-grade tumors. MRS is a useful problem-solving tool to grade gliomas; to differentiate them from other intra-axial lesions such as metastasis, anaplastic astrocytoma, oligodendroglioma, cerebral abscess, central nervous system lymphoma, encephalitis, radiation necrosis, tumefactive demyelination, and toxoplasmosis to delineate peritumoral microinfiltration, and to aid in preoperative mapping.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Glioblastoma. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics of Tumors of the Nervous System. Lyon, France: IARC; 2000. p. :29-39.
- [Google Scholar]
- Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235-41.
- [Google Scholar]
- Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging. 2012;12:423-36.
- [Google Scholar]





