Translate this page into:
Using of Fresh Cadaveric Cow Brain in the Microsurgical Training Model for Sulcal-Cisternal and Fissural Dissection
Address for correspondence: Prof. Cengiz Cokluk, Department of Neurosurgery, Medical Faculty, Ondokuzmayis University, Samsun, Turkey. E-mail: cengizcokluk@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The aim of this experimental study was to evaluate the feasibility of using fresh cadaveric cow brain as in a training model for microsurgical dissection of sulcus, cisterns, and fissure.
Methods:
Experimental microneurosurgical activities in this study were performed under the operating microscope. Bilateral sylvian cisterns, interhemispheric fissure, and hemispheric sulcus of the fresh cadaveric cow brain were used as an interested area for this experimental study. The dissection was continued reaching down to the floor of the cistern and total dissection of the middle cerebral artery inside the cisternal space. The suitability of a cow brain as a training model for sylvian fissure microdissection was evaluated as three groups; bad, good, and perfect.
Results:
Ten uncovered fresh cadaveric cow brains were used in this experimental feasibility study. The suitability of the experiment for training model was evaluated as bad in (1) 10% of the fresh cadaveric cow brains. The suitability was found as good in (6) 60% of the procedures. In the remaining (3) 30% of the brain dissection, the suitability of the experiment was evaluated as perfect.
Conclusion:
In conclusion, performing the sulcal, cisternal, and fissural dissection and protecting the neural and vascular tissue from the mechanical bruising effect of metallic microsurgical instruments are feasible as shown in this study. We believe that this training model will contribute to the practical micro-neurosurgery. Additionally, it provides adequate performance for the microsurgical intervention.
Keywords
Brain sulcal dissection
cisterns of brain
interhemispheric fissure
microneurosurgery
training of microsurgery
INTRODUCTION
Sharp and blunt dissection of the delicate neuroanatomical structures of the brain are commonly used in microneurosurgical practice. Some adjuvant-sophisticated systems may be used in the determination of the lesion location within the brain tissue. On the other hand, adequate microsurgical technique including dissection, separation, and retraction of the sulcus and/or cisternae is extremely important in the prevention of bruised effect of metallic microsurgical instruments. Microsurgical technique enables the neurosurgeon to dissect and expose the intracranial lesion such as aneurysms, vascular malformations, and tumors in a natural pathway within the subarachnoidal cisterns and sulcus.[1] Specifically, stereoscopic magnification with deep zoom and sharp focus provides the neurosurgeon adequate view of the pathology.[1] Arachnoid dissection is the real key to successful aneurysm operations.[1] Knowing the anatomy of subarachnoid cisternae is also important.
Specific microneurosurgical techniques such as proper use of the operative microscope, holding and grasping of the microneurosurgical instruments, proper microsurgical technique of the opening of the arachnoids membranes, safe and delicate neurovascular dissection, and careful and proper microdrilling of the cranial base bones should be learnt before operating on a patient.[12345] Theoretical knowledge, practical techniques, and microsurgical operative disciplines for the extremely protection of the delicate brain and related structures located within the cranium are mainly provided during the residency years of neurosurgical education.[16]
Many neurosurgeons interested in microneurosurgery try to gain some additional and advanced progress in improving their microneurosurgical ability including brain protection and delicate microneurosurgical techniques in laboratory training setting.[13456] Spending of time in experimental microsurgical laboratory to practice some kind of microsurgical models such as dissection and suturing of the rat external carotid artery, dissection and evaluation of the abdominal vena cava of rats, suturing of the plastic glove materials by using microforceps under the operating microscope, drilling and dissection of the some cadaveric bone materials are essential in improving and gaining of advanced microneurosurgical practical techniques.[13456] The aim of this experimental study was to evaluate the feasibility of microneurosurgical training model of cow sulcal, cisternal, and fissural dissection. Experimental findings, difficulties, practical methods, and suggestions were discussed in line with the existing literature.
MATERIALS AND METHODS
Experimental microneurosurgical activities in this study were performed under the operating microscope. Proximal and distal part of the Sylvian cistern and sulcal structure of cadaveric cow brain was used as an area of interest for this experiment. The uncovered cow brains were positioned supine on the operating platform. The microsurgical dissection procedure was started from the distal part of the sylvian cistern of the cow brains in the opening of the cisternal space [Figure 1]. The superficial direction of the origin of the middle cerebral artery from the internal carotid artery was used in the identification of the location of the sylvian cistern. Microscissor was used for cutting off the delicate arachnoids membrane overlying the sylvian cistern. Following the 10 mm opening of the arachnoids membrane, the dissection directed through the branch of the middle cerebral artery located inside the sylvian cistern. The dissection and separation was continued along the proximal part of the middle cerebral artery. It can be seen as the bifurcation of the middle cerebral artery at the middle part of the sylvian cistern. The main trunk, bifurcation, temporal, and frontal branch of the middle cerebral artery were identified. Figure 1 shows the microsurgical dissection of the sylvian cistern. The deep sulcal dissection was shown in Figure 2. The opposite side of the sylvian cistern was also dissected with same way. The second dissection and separation was continued with interhemispheric fissure. The arachnoid membrane over the interhemispheric fissure was cut using microscissor. The microsurgical dissection of the interhemispheric cistern is shown in Figure 3. Micro bayonet and the tip of the micro suction tube were used in the dissection and separation of the neural and vascular structures. The advanced and the third dissection were performed over the sulcus of the hemispheres. The delicate membrane over the sulcus was cut using microscissor. Using the tip of the micro bayonet, the brain sulcus was separated to dissect deep inside the sulcal structures. The microsurgical dissection of the sulcal, cisternal, and fissural space of the whole cow brain was shown in Figure 4. The suitability of the experimental process for microneurosurgical training model was evaluated within three groups as bad, good, and perfect. The criteria for perfect, good, and bad results were described as follows: in perfect result, the surgical specimens show no pial injury, cortical laceration, and separation following the surgical intervention. In good result, pial injury can be detected, but there is no cortical laceration. In bad result, pial and cortical injury, laceration, and separation may be present.

- The appearance of dissected sylvian cistern. MB: Micro bayonet, MCA: Middle cerebral artery, SC: Sylvian cistern, M2: Postbifurcation segment of the middle cerebral artery
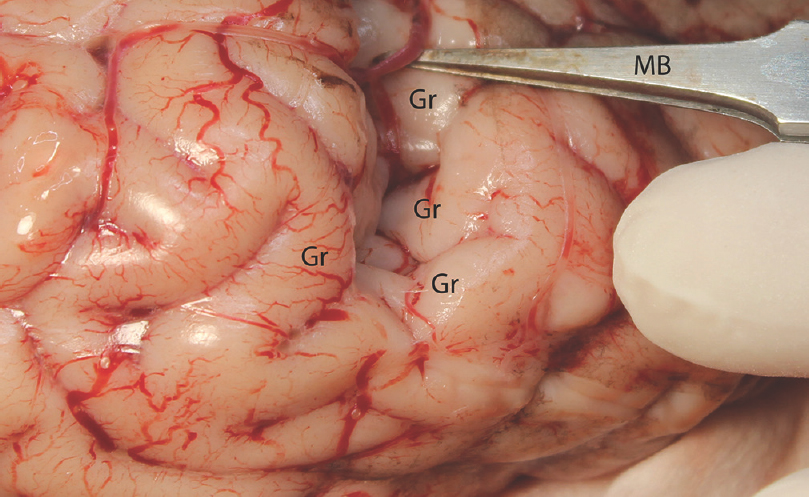
- The sulcal microdissection. MB: Micro bayonet, Gr: Gyrus
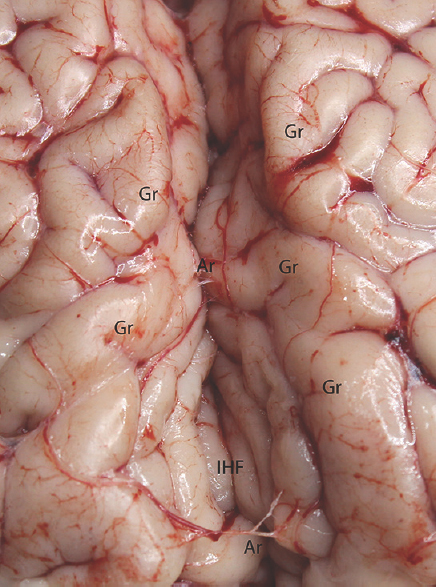
- Microdissection of the interhemispheric fissure. Gr: Gyrus, IHF: Interhemispheric fissure, Ar: Arachnoid membrane
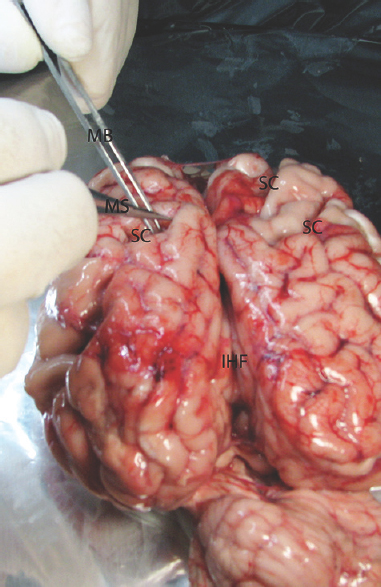
- The microsurgical dissection of the sulcal, cisternal, and fissural space of the whole cow brain. MB: Micro bayonet, MS: Microscissor, SC: Sulcus
RESULTS
Ten uncovered fresh cadaveric cow brains were used in this experimental feasibility study. The specimens were positioned with resembling of real operations to the middle cerebral artery for sylvian cistern dissection. On the other hand, the cow brains were positioned on the supine position for the sulcal and interhemispheric fissure dissection. Microscissor, micro bayonet, and tip of the metallic suction tube were used in the dissection of the extra-axial brain spaces. The sharp edge of the microscissor was used to cut and open the arachnoid membrane. Our experiences revealed that the arachnoid membrane of the cow brain is more delicate and thin in comparison with the arachnoid membrane of the human brain. It is necessary to extremely pay attention during the cutting of the arachnoid membrane in the avoiding of the neural tissue injury. Cottonoids may be used to separate brain tissue to protect from the blunt corner of the microsurgical instruments such as micro bayonet and microscissor. In general, a sufficient microsurgical technique should not cause any pial tissue injury.
The identification of the middle cerebral artery is easy to recognize and dissection. On the other hand, dissection of the venous structure is difficult in cadaveric cow brain specimens. The identification and separation of the interhemispheric fissure are easier in comparison with sylvian cistern. The sulcal dissection of the brain hemisphere is also important. The careful and delicate dissection and separation are necessary to open the sulcus. The suitability of the experiment for training model was evaluated as bad in (1) 10% of the fresh cadaveric cow brains. The suitability was found as good in (6) 60% of the procedures. In the remaining (3) 30% of the brain dissection, the suitability of the experiment was evaluated as perfect.
DISCUSSION
Regional microsurgical anatomy includes sulcus and cisterns. Microsurgical instruments should be well-known and recognized for a safe microneurosurgical intervention and gained practice.[7] It is also crucially important to use these instruments with appropriate microsurgical techniques.
Sylvian cistern is the most well-known neuroanatomical structure among the neurosurgeons. This cistern is transitional between the basal cisterns and the subarachnoid space over the convexities.[1] Sylvian cistern is a convenient corridor in reaching down to the cranial base such as sellar region, olfactory groove, optic chiasm, and carotid-cavernous structures. On the other hand, the most medial and inferior extent of the sylvian cistern is at the origin of the middle cerebral artery from the internal carotid. The sylvian cistern contains the middle cerebral artery and the origin of the lenticulostriate, temporopolar and anterior temporal arteries, the middle cerebral artery bifurcation, and the origins of the major branches.[8] The superficial and deep sylvian veins are also within the cistern. Dissection of the sylvian cistern should be started with opening of the arachnoid membrane overlying the cisternae distal to the middle cerebral artery bifurcation and superior to the temporal veins. After finding the distal part of the middle cerebral artery branches, the dissection should be directed to the bifurcation and proximal part of the middle cerebral artery. The separation and retraction of the frontal and temporal structures are necessary to reach the proximal part of the cistern.
Before a real operation performing on human beings, it is extremely necessary that understanding of the capability of some metallic surgical devices to be used in the microneurosurgical intervention. It is required for the person to develop his/her own abilities and to create integrated personal surgical techniques for the appropriate protection of brain.[34578] It is extremely imperative that surgical techniques should be repeated several times on appropriate models to successfully maintain and terminate microsurgical interventions.[7] Vascular end-to-end, end-to-side, side-to-side anastomosis, aneurysm clipping, and sylvian fissure dissection may be practiced using similar microsurgical training models.[24568]
In this experimental model, fresh cadaveric cow brains were evaluated for its suitability as a training model for fissural, sulcal, and cisternal dissection. An appropriate and successful model should have some similarities with the represented human organ or system. Ethical issues with live models in addition to the above disadvantages also pose problematic limitations in experimental practice.
When we think of all these features together, the cow brain should be regarded as a suitable model in the experimental microneurosurgical training for sulcal and cisternal dissection. There are a few differences between the human and cow brain. The human brain is larger in size and shape when compared to the cow's brain. The cow brain does not have as many gyri and sulci when compared to human brain. The human brain of an adult weighs about 1200–1500 g, and 10–20 cm in length. A cow's brain is elongated in shape, whereas a human brain is rounded. The human brain is not only larger, but also heavier than a cow's brain, because it is only 400–600 g compared to the human brain. However, there are some other differences in human and cow brains, but almost all mammals’ brain are similar. Except some anatomical differences, the location of the interhemispheric sulcus and the arachnoid membrane location have the same characteristic feature between human and cow brain. In this experimental model, the similar microsurgical instruments were used during dissection, separation, and distraction of the brain. Microscissor, the tip of the micro aspirator, and micro bayonet were used in the dissection and separation of the neural and vascular structures.
Sulcus and/or cisternae dissection and separation in the microsurgical brain operation are commonly used in microneurosurgical practice. Performing of the finely locate the lesion using neuroradiological images can be performed during the surgical intervention. Following the cisternae dissection and/or splitting of the brain parenchyma, operative corridor may be reached down and pathology localized or located inside sulcus and cisterns. Repetitive training of the sulcal and cisternal dissection in the models like ours is crucial in improving safety in microsurgical intervention. This experimental study revealed that cow brain is a suitable model for training in fissural, sulcal, and cisternal dissection. The suitability of the experiment for training model was evaluated as bad in (1) 10% of the fresh cadaveric cow brains. The suitability was found as good in (6) 60% of the procedures. In the remaining (3) 30% of the brain dissection, the suitability of the experiment was evaluated as perfect.
CONCLUSION
To sum up, safe surgical interventions require protecting of the brain tissue and neurovascular structures. Thus, it is extremely necessary to perform dissection and separation on neurovascular on training models before real practices on humans. Cow's sulcal, cisternal, and fissural training model is feasible as shown in this experimental study. We believe that this training model will contribute to the practical microneurosurgery in various terms. Primarily, it helps in protecting neurovascular tissue. In addition, it provides adequate performance for the microsurgical intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Microneurosurgery. New York: Georg Thieme Verlag Stutgard; 1984. p. :215.
- Off-the-job microsurgical training on dry models: Siberian experience. World Neurosurg. 2014;82:20-4.
- [Google Scholar]
- Microneurosurgical training model in fresh cadaveric cow brain: A laboratory study simulating the approach to the circle of willis. Surg Neurol. 2006;66:100-4.
- [Google Scholar]
- Laboratory training in the retrosigmoid approach using cadaveric silicone injected cow brain. Br J Neurosurg. 2013;27:812-4.
- [Google Scholar]
- Training models for vascular microneurosurgery. Acta Neurochir Suppl. 2011;112:115-9.
- [Google Scholar]
- Microsurgical training model for residents to approach to the orbit and the optic nerve in fresh cadaveric sheep cranium. J Neurosci Rural Pract. 2014;5:151-4.
- [Google Scholar]
- Maintaining microneurosurgical ability via staying active in microneurosurgery. Minim Invasive Neurosurg. 2007;50:324-7.
- [Google Scholar]
- Microneurosurgical skills training. J Neurol Surg A Cent Eur Neurosurg. 2016;77:146-54.
- [Google Scholar]






