Translate this page into:
The Utility of Preoperative ACTH/Cortisol Ratio for the Diagnosis and Prognosis of Cushing's Disease
Address for correspondence: Dr. Alev Selek, Department of Endocrinology and Metabolism, Faculty of Medicine, Kocaeli University, Umuttepe Kocaeli 41380, Turkey. E-mail: alevselek@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose:
Cushing's syndrome (CS) is a rare disease having diagnostic difficulties. Many diagnostic tests have been defined but none of these are diagnostic alone. Determination of the cause is another problem which sometimes requires more sophisticated and invasive procedures. Therefore, we aimed to evaluate the utility of pretreatment plasma adrenocorticotropic hormone (ACTH)/cortisol ratios in patients with confirmed endogenous CS for the diagnosis and differential diagnosis of CS.
Materials and Methods:
This retrospective evaluation included 145 patients with the diagnosis of CS, 119 patients with Cushing's disease (CD), and 26 patients with ACTH-independent CS (AICS), in a university hospital. Furthermore, 114 individuals in whom CS diagnosis was excluded with at least one negative screening test were enrolled to the study as control group. The clinical, laboratory, imaging, postsurgical pathologic records and also clinical follow-up data of all patients were evaluated.
Results:
The median basal ACTH/cortisol ratio of the patients with CD was significantly higher than AICS and controls. A cutoff ACTH/cortisol ratio >2.5 was found to be diagnostic for CD with 82% specificity and 63% sensitivity. Among CD group, patients with recurrent disease had higher preoperative ACTH levels and ACTH/cortisol ratio than patients with sustained remission. Furthermore, these patients had more invasive, atypical, and larger tumors.
Conclusion:
An ACTH/cortisol ratio >2.5 would be beneficial to diagnose CD together with other diagnostic tests. It is a simple test with no additional cost. Higher ratios might be related with larger, invasive, and atypical adenoma and also might be helpful to predict recurrence.
Keywords
Adrenocorticotropic hormone/cortisol ratio
Cushing's disease
diagnosis of Cushing's syndrome
recurrent Cushing's disease
INTRODUCTION
The diagnosis of Cushing's syndrome (CS) presents clinical challenges. Many diagnostic tests have been defined in this regard with variable sensitivity and specificity. However, none of these tests are diagnostic alone. Therefore, combination of these tests is usually preferred for accurate diagnosis of endogenous CS. A screening laboratory evaluation for CS should be considered in any patient with signs and symptoms of excessive cortisol secretion. The recommended diagnostic tests in patients with suspected CS are late-night salivary or plasma cortisol, 24 h urine free cortisol (UC), and morning cortisol levels after low-dose dexamethasone suppression (LDDS). The diagnosis of CS is established when at least two different screening tests are unequivocally abnormal.[1] Once the diagnosis is established, the cause of the hypercortisolism should be identified. To determine whether the hypercortisolism is adrenocorticotropic hormone (ACTH) dependent or ACTH independent, morning plasma ACTH concentration should be assessed at least 2 times. Plasma ACTH < 5 pg/mL in a hypercortisolemic patient is evidence of ACTH-independent disease while an ACTH concentration above 20 pg/mL suggests that cortisol secretion is ACTH dependent. ACTH levels between 5 and 20 pg/mL are less definitive, but usually indicate an ACTH-dependent cause.[1]
Majority of the cases with ACTH-dependent CS are caused by ACTH-secreting pituitary adenoma.[2] Suppression of cortisol during high-dose dexamethasone administration and increases in plasma ACTH and serum cortisol levels after CRH stimulation are consistent with the diagnosis of Cushing's disease (CD). In cases with pituitary adenoma < 5 mm, inferior petrosal sinus sampling is indicated to show a high petrosal sinus to peripheral plasma ACTH gradient.[1] Despite these diagnostic tests, there are still some cases with indeterminate results causing diagnostic challenge.
ACTH values are positively correlated with the size of the pituitary adenoma in patients with CD.[345] However, cortisol levels are not usually increased as high as ACTH levels. Lower cortisol/ACTH ratios have been found in patients with CD harboring macroadenoma (MAC) than microadenoma (MIC) cases.[4] Although ACTH levels are usually used to determine the cause of CS, ACTH/cortisol ratio might be useful for both diagnosis and establishing the cause. Beyond indicating pituitary directed disease, higher ACTH/cortisol ratio might be useful for the diagnosis of CS. For this purpose, we assessed the pretreatment plasma ACTH/cortisol ratios in patients with confirmed endogenous CS with pituitary or adrenal causes and control individuals without CS. We aimed to evaluate the utility of these measurements for the diagnosis and differential diagnosis of endogenous CS.
MATERIALS AND METHODS
Patients
This retrospective evaluation included 165 patients with the diagnosis of CS between 2007 and 2016 in a university hospital. Patients without confirmatory clinical, laboratory, and radiologic data for CS and patients who were not treated surgically were excluded. Finally, this study included 119 patients with CD and 26 patients with ACTH-independent CS (AICS). Furthermore, 114 individuals in whom CS diagnosis was excluded with at least two negative screening tests were enrolled to the study as control group.
Data collection
The clinical, laboratory, imaging, and postsurgical pathologic records and also clinical follow-up data of all patients were obtained from our outpatient clinic. Preoperative diagnostic tests were reevaluated in all patients and postoperative disease activity, remission, and recurrence status were also noted.
ACTH, p53, and Ki-67 immunohistochemical staining data of the pituitary tumor tissues and Weiss score of the adrenocortical tumors were obtained from postoperative pathology reports from the pathology department. All pituitary and adrenocortical tumors were evaluated by a single endocrine pathologist.
All patients with CD underwent magnetic resonance imaging (MRI) preoperatively and postoperatively also whenever needed on follow-up. Patients with AICS had preoperative adrenal gland MRI, and if remission was achieved, no further MRI was needed. Tumor features including size, cavernous sinus invasion, sellar erosion, and optic chiasm compression were evaluated on pituitary MRI. Visible lesions with a diameter of ≥10 mm were defined as MAC and MIC was defined as visible lesions < 10 mm of diameter or in cases with MRI negative/doubtful but postoperative remission was achieved or tumor identification by the neurosurgeon was compatible with MIC. On adrenal imaging, only size was noted as all tumors were benign adenoma.
Hormonal evaluation for diagnosis and remission
Preoperative basal ACTH and cortisol levels of both patient groups and basal hormonal levels of control group were evaluated. Basal ACTH/cortisol ratio was also calculated and compared between CD and control group. Twenty-two of 26 patients in AICS had undetectable basal ACTH levels (<5 pg/mL); therefore, ACTH/cortisol ratio was not calculated. ACTH and cortisol levels were recorded by calculating the average of the two-morning values closest to the operation. Serum cortisol was measured by immunoenzymatic assay (UniCel DxI 600, Beckman Coulter, USA) and ACTH was measured with chemiluminescent immunometric assay (Immulite 2000cXPi, Siemens, USA).
Hypercortisolism was confirmed by at least two positive screening tests; increased free cortisol excretion (fold increase from upper limit of normal [×ULN] was calculated) or failure to suppress plasma cortisol after low dose of dexamethasone or increased midnight cortisol levels. The pituitary origin of the hypercortisolism was established on the basis of ACTH concentrations above 10 pg/mL, adequate suppression of cortisol during high-dose dexamethasone administration (>50% suppression from baseline levels), or a high petrosal sinus to peripheral plasma ACTH gradient (if test was available). In some cases, CRH stimulation test was also available. Thorax and abdomen computerized tomography were also seen in patients with ACTH >150 pg/mL to rule out possible ectopic causes (10 patients). If ACTH was < 10 pg/mL, directly adrenal imaging was performed.
Postsurgical remission was defined as a low (<5 pg/mL) or undetectable serum cortisol level at first 48 h after pituitary surgery. CD cases were operated without surgical steroid coverage and monitored closely for adrenal insufficiency symptoms and signs postoperatively. In case of adrenal insufficiency, blood samples were taken for cortisol levels and steroid treatment was started. In patients with AICS, remission was assessed after cessation of steroid treatment. Sustained remission in CD was decided in case of clinical adrenal insufficiency, the need for glucocorticoid replacement, disappearance of clinical hypercortisolism, and normal UC levels and successful suppression of cortisol levels after low-dose dexamethasone (after glucocorticoid withdraw) for a minimum of 6 months after pituitary surgery. Patients who failed to meet these criteria were classified as having persistent CD. Recurrence was defined as the reappearance of clinical symptoms and signs of hypercortisolism and at least two screening tests pointing to CD activity >6 months after surgery. Ki 67 percentage and p53 positivity were also assessed. An adenoma with Ki 67 index ≥2% and p53 positivity was defined as atypical adenoma.
Statistical analysis
All statistical analyses were performed using IBM SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA). Kolmogorov–Smirnov tests were used to test the normality of data distribution. Continuous variables were expressed as mean ± standard deviation, median (25th–75th percentiles), and categorical variables were expressed as counts (percentages). Differences between the groups were analyzed by Student's t-test and one-way ANOVA for numerical variables with normal distribution and Mann–Whitney U-test, for numerical variables with nonnormal distribution and Pearson Chi-square test for categorical variables. The relationship between numerical variables was evaluated by Spearman Correlation Analysis. P < 0.05 was considered statistically significant for significance.
The study protocol was approved by the local ethics committee. Informed consent had been taken from all study subjects and controls. There is no conflict of interest for any authors.
RESULTS
Clinical characteristics
The demographic characteristics of all study groups are shown in Table 1. The mean age at the diagnosis was similar in CD and control group while AICS patients were slightly older than CD (40 vs. 49.4 years, P = 0.01). There was female predominance in all study groups. Patients with < 3 months of follow-up were not included in the recurrence and follow-up analysis. The mean follow-up period was 32.5 months (range 3–105) for 86 patients in CD group. All of the patients in the AICS group were attended to clinical follow-up with a mean of 34 months (range 3–108).

Hormonal data
Adrenocorticotropic hormone/cortisol ratio
Preoperative basal ACTH and cortisol levels of both patient groups and basal hormone levels of control group were defined in Table 1. Basal ACTH/cortisol ratio of CD and control group were calculated and median levels were also shown in Table 1. Basal ACTH levels were significantly higher in CD group than control group (P < 0.001). Almost all patients with AICS had basal ACTH < 10 pg/mL and 22 of them had undetectable levels (<5 pg/mL); therefore, ACTH/cortisol ratio of AICS group was not calculated. Basal cortisol levels were also high in both patient groups than controls and highest in CD group (P < 0.001, P = 0.03 respectively). The median ACTH/cortisol ratio of the patients with CD was 3.2 and significantly higher than control group, 1.8 (P < 0.001). We further evaluated the utility of ACTH/cortisol ratio for the diagnosis of CD. Among CD and controls, an ACTH/cortisol ratio >2.5 was found to be diagnostic for CD with 82% specificity and 63% sensitivity [Figure 1]. As basal ACTH levels were < 10 pg/ml in 96% of patients with AICS, an ACTH/cortisol ratio >2.5 also completely excludes AICS in an hypercortisolemic patient.
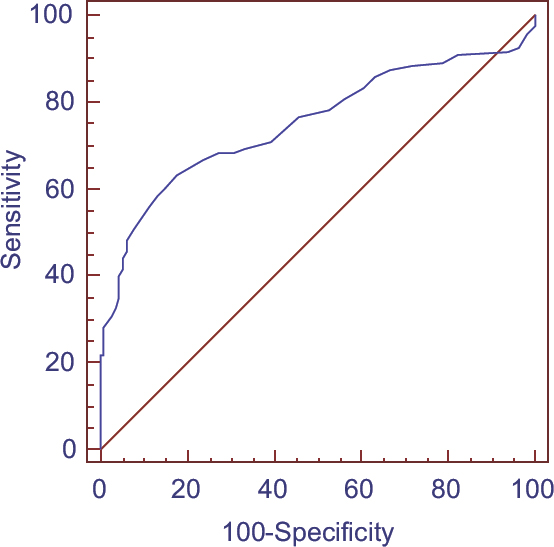
- Receiver operating characteristic analysis of adrenocorticotropic hormone/cortisol ratio
ACTH/cortisol ratio of the patients with CD was positively correlated with the size of the adenoma and Ki 67 expression (r: 318, P < 0.001 and r: 218, P = 0.018, respectively). However, there was no relation between ACTH/cortisol ratio and diagnostic tests including midnight cortisol, urinary free cortisol, and LDDST.
Remission and recurrence
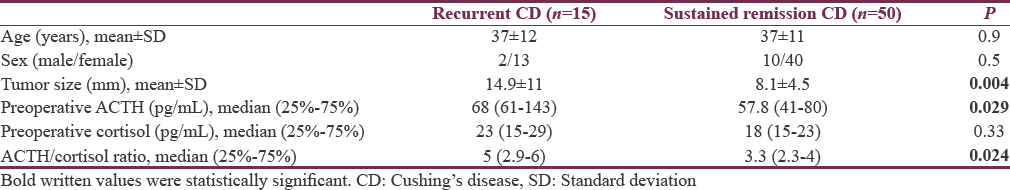
Postoperative remission was achieved in 81 (68%) patients in our CD cohort; 65 of these patients had complete follow-up data. Among 65 patients, 15 (23%) patients had recurrent CD, 10 of them were reoperated and 5 of them had stereotaxic radiosurgery. Patients with recurrent disease had higher adenoma size, preoperative ACTH levels, and ACTH/cortisol ratio than patients with sustained remission (P = 0.004, P = 0.029 and P = 0.024 respectively) [Table 2]. The mean age and sex were similar in patients with and without recurrent disease. However, the tumors of the patients with recurrence had more cavernous sinus invasion and pathologic atypia (P = 0.01 and P = 0.005 respectively) [Table 3].

Adenoma size
Among CD patients, 66 (55%) had MIC and 21 patients (17%) had cavernous sinus invasion preoperatively. The gender and mean age of the patients with microadenoma and MAC, with or without cavernous sinus invasion were similar. Postoperative remission was achieved less frequently in patients with cavernous sinus invasion and MAC (P = 0.008 and P = 0.02 respectively). Basal ACTH and ACTH/cortisol ratio were significantly higher in MAC patients (P < 0.001) [Table 4]. Furthermore, recurrent disease was seen more frequently in patients with MAC than MIC (35% vs. 8%, P = 0.019). Pituitary adenoma size was positively correlated with Ki 67 index and cortisol levels after LDDS (r: 289, P = 0.02 and r: 238, P = 0.03, respectively).

Patients with cavernous sinus invasion and atypical adenoma had significantly higher preoperative ACTH and ACTH/cortisol ratio while patients with postoperative remission had lower ratio [Table 4]. The adjusted ACTH/cortisol ratio analysis according to adenoma size was still significantly higher in recurrent disease and pathologic atypia (P = 0.02, and P = 0.002, respectively).
DISCUSSION
In the current study, we evaluated the ACTH/cortisol ratio among patients with different etiologies of CS and controls to demonstrate diagnostic accuracy. ACTH/cortisol ratio of the patients with CD was significantly higher than participants without CS. Furthermore, this ratio was < 1 in all of the AICS patients. These results showed ACTH/cortisol ratio might be a simple and useful test for the diagnosis of ACTH-dependent CS. In receiver operating characteristic curve analysis for the utility of ACTH/cortisol ratio in the diagnosis of CD, an ACTH/cortisol ratio >2.5 was found to be diagnostic with 82% specificity and 63% sensitivity. Actually, this cutoff would be diagnostic for ACTH-dependent CS covering also ectopic causes. In our study, ectopic cases were not included for analysis because there were only two cases.
Cortisol is secreted in a pulsatile manner accompanying the pulsatile secretion of ACTH. Therefore, ACTH/cortisol ratio should be in a narrow range in a patient with intact ACTH-cortisol axis. Loss of negative cortisol feedback at the pituitary level is a key feature of CD and represents the main mechanism of the diagnostic tests.[1] Normally, administration of an exogenous glucocorticoid leads to suppression of endogenous cortisol and ACTH production, whereas in CD, there is inadequate suppression.[6] It can be predicted that the loss of negative cortisol feedback would also impair ACTH and cortisol pulsatility causing increased ACTH/cortisol ratio in CD.
The diagnosis of CD was a dilemma for decades. The expected improvement in this regard could not be achieved over time; it is still a challenge. The clinical sign and symptoms are variable and usually nonspecific. Although easy bruising, facial plethora, proximal muscle weakness, and purple striae >1 cm wide are the most discriminating signs for CS; none of these are pathognomonic.[1] As a result, the diagnosis must be confirmed by biochemical tests. Unfortunately, there is no single test to be used for diagnosis, combination of the tests such as late-night salivary or plasma cortisol, 24 h urine free cortisol, and morning cortisol levels after LDDS are usually used. However, in some cases with conflicting results, more complex tests with corticotropin-releasing hormone and desmopressin are suggested.[1] For this purpose, calculated ACTH/cortisol ratio is thought to be an alternative simple test which might strengthen the diagnosis of CD.
The current study had also shown that ACTH/cortisol ratio was also related with some clinical and prognostic markers. In our study, we found a positive correlation between the maximum diameters of pituitary lesions and plasma ACTH, similar to previously published data.[457] Moreover, ACTH/cortisol ratio was also evaluated and found positively correlated with tumor size and Ki 67 expression. Patients with MAC, cavernous sinus invasion, and atypical adenoma had higher ACTH/cortisol ratio. This group of patients also had lower postoperative remission and higher recurrence rate. Therefore, higher ACTH/cortisol ratio might predict poorer prognosis. Higher concentrations of ACTH and lower serum cortisol/ACTH ratio in CD with MAC were found in some studies.[345] Machado et al. had also showed a high prevalence of invasion, mainly in the cavernous sinus, in MAC patients with no postsurgical remission.[4]
Recurrence and persistence are another two difficult entities in CD. CD is a serious condition with an excess mortality, and unfortunately, the risk of recurrence after initial surgical cure is high and necessitates lifelong monitoring.[8910] In most of studies, postoperative ACTH and cortisol levels and tumor size were found to be related with remission, but there is only limiting data to predict which patients will have recurrence. Higher preoperative ACTH levels have been shown to predict disease recurrence[11] and mortality[12] during follow-up. In the current study, the recurrence rate was 23% consistent with the literature.[1112] Patients with recurrence had higher tumor size, preoperative ACTH levels, and ACTH/cortisol ratio than patients with sustained remission. Furthermore, cavernous sinus invasion and pathologically atypical adenoma were seen more frequently in these patients. The adjusted ACTH/cortisol ratio analysis according to adenoma size was still significantly higher in recurrent disease and pathologic atypia. These findings suggested that invasive, atypical MACs would be at higher risk for recurrence. A simple and no time-consuming calculation for preoperative ACTH/cortisol ratio would be a clue to predict recurrence.
The current study was a retrospective study which limits the accuracy. However, all preoperative biochemical analyses of the patients were done according to our routine protocol that would reduce possibility of mistakes. Clinical follow-up of some CD patients were missing; therefore, recurrence and sustained remission were only assessed in 86 (72%) of the patients. Although mean clinical follow-up was 32.5 period, there were some patients with shorter period in CD cohort. Higher number of patients and longer follow-up would yield more accurate results.
CONCLUSION
An ACTH/cortisol ratio >2.5 would be beneficial to diagnose CD together with other diagnostic tests. It is a simple test with no additional cost. Higher ratios might be related with higher tumor size, invasive, and atypical adenoma. Furthermore, this test might be helpful to predict recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The diagnosis of Cushing's syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-40.
- [Google Scholar]
- AACE/ACE disease state clinical review: Medical management of Cushing disease. Endocr Pract. 2014;20:746-57.
- [Google Scholar]
- Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005;90:4963-9.
- [Google Scholar]
- Negative correlation between tumour size and cortisol/ACTH ratios in patients with Cushing's disease harbouring microadenomas or macroadenomas. J Endocrinol Invest. 2016;39:1401-9.
- [Google Scholar]
- Cushing's disease: A comparison of pituitary corticotroph microadenomas and macroadenomas. Eur J Endocrinol. 1998;138:153-9.
- [Google Scholar]
- The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647-72.
- [Google Scholar]
- Comparisons between macroadenomas and microadenomas in Cushing's disease: Characteristics of hormone secretion and clinical outcomes. J Korean Med Sci. 2009;24:46-51.
- [Google Scholar]
- Incidence and late prognosis of Cushing's syndrome: A population-based study. J Clin Endocrinol Metab. 2001;86:117-23.
- [Google Scholar]
- Transsphenoidal microsurgery for Cushing's disease: Initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89:6348-57.
- [Google Scholar]
- Adrenocorticotropic hormone levels before treatment predict recurrence of Cushing's disease. J Formos Med Assoc. 2017;116:441-7.
- [Google Scholar]
- Predictors of mortality and long-term outcomes in treated Cushing's disease: A study of 346 patients. J Clin Endocrinol Metab. 2013;98:1022-30.
- [Google Scholar]






