Translate this page into:
The Role of Clinical Variables and VKORC1 Polymorphism in Efficacy and Stability of Acenocoumarol in Neurological Patients
Address for correspondence: Dr. Usha Kant Misra, Department of Neurology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareily Road, Lucknow - 226 014, Uttar Pradesh, India. E-mail: drukmisra@rediffmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To analyze the clinical importance of VKORC1 polymorphism and its correlation with stability of oral anticoagulation.
Patients and Methods:
In a hospital-based study, the patients on oral anticoagulant (OAC) were included during 2013–2016. The patients received OAC for cardioembolic stroke, cerebral venous sinus thrombosis (CVST), and prevention of deep vein thrombosis (DVT). Demographic, clinical, and neurological findings were recorded. Stability of anticoagulation was determined by percentage of time international normalized ratio (INR) values were in therapeutic range. Time in therapeutic range (TTR) >65% was defined as stable and <65% was defined unstable. VKORC 1 polymorphism was studied by polymerase chain reaction and correlated with daily dose of OAC and stability of INR.
Results:
A total of 157 patients with a median age of 40 years were included in the study. Ninety-two patients received OAC for secondary stroke prevention, 62 for CVST, and 3 for DVT. Out of 2976 INR reports, 1458 (49%) were in the therapeutic range, 997 (33.1%) were below the therapeutic range, and 521 (17.5%) were above the therapeutic level. Stable INR was obtained in 75 (47.77%) patients which was improved by drug modification in 3 and dietary adjustment in 12 patients. VKORC1 polymorphism revealed GG genotype in 127 (80.9%), GA genotype in 22 (14%), and AA genotype in 8 (5.1%) patients. Therapeutic range of INR was seen in 49%, below therapeutic range was seen in 31.5%, and above in 17.5%.
Conclusion:
VKORC1 polymorphism was related to mean daily dose of OAC but not to the stability of INR.
Keywords
Cardioembolic stroke
international normalized ratio stability
oral anticoagulation
Vitamin K antagonist
VKORC1 gene polymorphism
INTRODUCTION
Vitamin K antagonists (VKAs) block the function of Vitamin K epoxide reductase complex in the liver, leading to depletion of reduced form of Vitamin K that serves as a cofactor for gamma carboxylation of Vitamin K-dependent coagulation factors.[1] VKAs are used for the prevention of cardioembolic stroke (CES) and other disorders. In spite of new oral anticoagulants (OAC), VKAs continue to be important, especially in the developing countries. Anticoagulation generaly is used for the prevention of CES and cerebral venous sinus thrombosis (CVST) prophylaxis.[23] These conditions are associated with multiple comorbidities such as diabetes mellitus, hypertension, myocardial infarction, dilated cardiomyopathy (DCMP), atrial fibrillation, and multiple drugs with potential of drug interactions. It is, therefore, difficult to achieve and maintain optimal international normalized ratio (INR). Target INR is kept in the range of 2–3.5 in most of the patients. Meta-analyses have shown that time in therapeutic range (TTR) ranged from 29% to 75% and percentages of INR values were in the recommended range of 34%–84%.[4] Difficulty in attaining target INR is either attributed to sensitivity or resistance to OAC which is determined by a number of variables such as co-medications, diet, age, and genetic variation. VKORC1 is the most important gene in this regard. VKORC1 gene encodes Vitamin K epoxide reductase enzyme. VKA acts by inhibiting this enzyme, reducing the reduced form of Vitamin K, which is required as a cofactor in gamma carboxylation of Vitamin K-dependent coagulation factors. There are many single-nucleotide polymorphisms (SNPs) of VKORC1; these include 3462C>T or 8773C>T, 2255C>T or 7566C>T, 1542G>C or 6853G>C, 1173C>T, 6009C>T, or 698C>T. The SNPs 1173C>T in intron 1 and 3730G>A are in linkage disequilibrium with 1639G>A in the promoter region. Polymorphism in this region leads to abnormal expression of the enzyme which correlates with the dose of OAC.[5] Several studies have shown that the VKORC1 gene polymorphism is the most important predictor of warfarin dose requirement.[67] Thus, by predicting dose requirement, we hypothesize that genotyping may also affect the stability of INR control. A number of studies on VKORC1 genetic polymorphism have been done in cardiology, internal medicine patients, or in anticoagulation clinics. Few studies have been done in neurological patients. Neurological patients have special limitations. Often, they are dependent on caregivers for monitoring. The present study has been undertaken to study the VKORC1 gene polymorphism and to correlate it with the dose of OAC and stability of INR in a cohort of neurological patients.
PATIENTS AND METHODS
Consecutive patients on OAC attending the neurology service of a tertiary care teaching institute in India between March 2013 and February 2016 have been included. The patients were personally examined and followed up by the authors. The patients who were prescribed OAC for the first time were prospectively followed up. The patients who were already on OAC were also prospectively followed up and their clinical data were retrospectively evaluated. The study was approved by the Institute Ethics Committee. Informed consent was given by the patients or their authorized representatives.
Exclusion criteria
Patients with pregnancy, hepatic or renal failure, multiorgan dysfunction, or unwilling to participate in the study were excluded.
Methods
A detailed medical history taking and clinical examination were done. Patients’ demographic information including age, gender, diet, ethnicity, and religion were noted. Presence of anemia, edema, petechial hemorrhage, epistaxis, hematemesis, and melena was inquired. Their pulse rate and rhythm were noted and abnormalities in cardiac examination were noted. Neurological examination included level of consciousness, mental status, muscle power, tone, tendon reflexes, sensations, and co-ordination. Electrocardiography, Doppler echocardiography, and magnetic resonance imaging with venography were done as per clinical indication following which the patients were categorized into those with rheumatic heart disease (RHD), prosthetic valve, DCMP, cerebral venous sinus thrombosis (CVST), deep vein thrombosis (DVT), and miscellaneous disorders. Adequacy of anticoagulation was defined by the INR value based on recommended therapeutic range for different conditions. Advice about dietary and drug interaction with OAC was given at the first visit. On follow-up, adequacy of OAC was defined based on INR value. In general, the starting dose of acenocoumarol was 2 mg/day and the dose was adjusted based on INR value. The INR was measured every 3 days until two consecutive INR values were in the therapeutic range. Thereafter, INR was repeated after 1 week; if INR was in therapeutic range, then it was repeated monthly. Patients with suboptimal INR were inquired about diet and OAC dose and compliance. These factors if found abnormal were corrected and INR was repeated after 1–2 weeks. If INR was suboptimal even after correcting these factors, the dose of OAC was increased by 10%–20%. The INR values in the initial 2 weeks of OAC treatment and those values after temporary discontinuation of drug due to surgical procedures or other conditions were excluded. INR was done in the WHO-accredited laboratory, but patients from far off place were advised to get the prothrombin time INR test locally. Abnormal reports from outside were verified from our laboratory before modifying the OAC dose. The anticoagulation status was categorized into three groups based on the recommended INR value for the respective disease as optimal (in the therapeutic range), suboptimal (below the therapeutic range), and above the therapeutic range. Stable INR was defined by three consecutive INR values in therapeutic range at least 1 month apart without changing the dose of OAC. Patients who had TTR >65% were defined as stable and <65% were considered unstable.[8] Polypill therapy was defined if the patient was taking ≥4 drugs. During the follow-up period, complications were noted and the corresponding INR values were recorded.
VKORC1 polymorphism
DNA extraction
Two milliliters of venous blood was collected in ethylenediaminetetraacetic acid (EDTA) vials from each patient for genomic DNA extraction using the standard salting-out method.[9] The DNA quantity was checked using Nano-Drop Analyzer spectrophotometer (NanoDrop 2000ThermoScientific, Wilmington, DE, USA). Absorbance ratio of DNA at 260–280 nm was between 1.7 and 1.9 and the quality of DNA was checked by agarose gel electrophoresis. Genotyping for polymorphisms of VKORC1 (-1639G>A) was performed by polymerase chain reaction (PCR) using Veriti 96-well thermal cycler (Applied Biosystem; Life Technologies Corporation, Carlsbad, CA, USA) and restriction fragment length polymorphism (RFLP).[10] PCR was used to amplify VKORC1 gene segments from genomic DNA extracted from patients using the following set of primers: forward - 5’ATCCCTCTGGGAAGTCAGC-3’ and reverse - 5’CACCTTCAACCTCTCCATCC-3’ (Eurofins genomics India Pvt Ltd., India). PCR condition was as follows: 94°C for 5 min followed by 35 cycles at 94°C for 45 s, 56°C for 1 min, and 72°C for 2 min, and by final extension at 72°C for 7 min. In a reaction mixture of 25 μL, template DNA of patients was 5 μL, 0.5 μL dNTP mix, 0.4 μL Taq DNA Polymerase, 1.3 μL of buffer, 1.0 μL of each forward and reverse primers, and 15.8 μL of MilliQ water were added per reaction. The PCR products were run on 2% agarose gel stained with ethidium bromide (Etbr) in Tris Borate EDTA (pH 8.2) buffer. Further, Restriction Fragment Length Polymorphism (RFLP) was done using restriction enzyme Nci1 (New England Biolabs); 8 μL of PCR products in a reaction mixture of 15 μL consisting 1 μL RE Nci1, 1 μL digestion buffer, and 5 μL MilliQ water were digested at 37°C in a water bath for not <16 h. The digested amplicons were visualized on agarose gel under ultraviolet irradiation. Amplicon size for VKORC1 was 636, and wild and minor alleles were 522 and 114, respectively.
Statistical analysis
The relationship of anticoagulation status with demographic and clinical variables was evaluated using Chi-square test for categorical and independent or Mann–Whitney U-test for continuous variables. The factors influencing the stability of INR were evaluated by univariate followed by multivariate analysis. The factors which affected mean daily dose were evaluated by generalized linear model. The variable was considered significant if two-tailed P < 0.05. The statistical tests were done using SPSS, Version 16.0. Chicago, SPSS Inc.
RESULTS
During the study period, 170 patients were enrolled who were on OAC. Thirteen patients were excluded because eight patients were lost to follow-up. Samples of five patients were lost. The result is therefore based on 157 patients.
Mean age of the patients was 40.9 ± 15.0 years with range between 3 and 84 years [Table 1]. There were 115 patients (73.2%) who were ≤50 years of age. Eighty-two patients (52.2%) were males. Seventy-four patients (47.1%) resided in rural area. Fifty-two (33.1%) patients had education of ≤8th standard. Eighty-nine patients (56.7%) were vegetarian. Ninety-two patients (58.6%) were taking OAC for CES, 62 (39.5%) patients for CVST, and three patients (1.9%) for DVT. Out of the 92 patients, 58 patients had RHD with valvular heart disease, 19 patients had prosthetic heart valve, 12 had DCMP, 3 had myocardial infarction, and 37 patients had atrial fibrillation. Three patients received OAC for DVT which they developed during hospitalization. Thirty-one patients (19.7%) were addicted to intoxicants, 15 patients (9.6%) consumed alcohol, 13 (8.2%) were smokers, and 20 (12.7%) were tobacco chewers. Thirteen patients (8.3%) had diabetes mellitus and 10 (6.3%) were hypertensives [Table 1]. A total of 120 patients were followed up prospectively, 36 patients had both prospective and retrospective data and 1 patient only had retrospective data. During follow-up, 2976 INR reports were done. Out of which, 1458 (49%) were in optimal range, 997 (33.5%) were in suboptimal range, and 521 (17.5%) were above therapeutic range. A total of 2147 INR reports (72.14%) were done prospectively, out of which 1099 reports (51.2%) were in optimal range. A total of 829 (27.86%) were done retrospectively, out of which 359 reports (43.3%) were in optimal range.
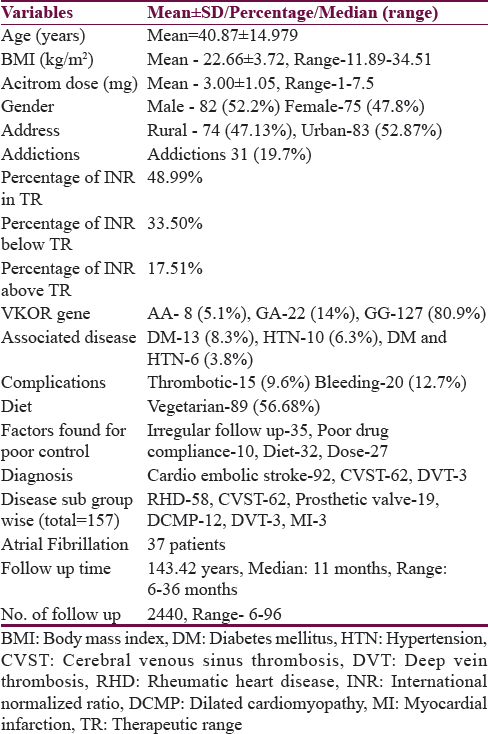
Stability of international normalized ratio
Stable INR was present in 75 patients (47.8%) only. Twelve patients achieved stable INR after dietary modification. In nine patients, the INR was achieved in stable range after adjusting the dose of OAC (increased in six and decreased in three). The dose of OAC was reduced in three patients who were changed from valproate to levetiracetam and in seven patients in whom atorvastatin was stopped. Six patients were sensitive to OAC and needed 1 mg or less acenocoumarol while seven patients were resistant needing more than 5 mg of acenocoumarol for achieving optimal INR value.
On univariate analysis, stability of INR was related to urban residence, male gender, absence of associated hypertension or diabetes, polypill use, digoxin use, and proton pump inhibitor (PPI) intake. Stability of INR was not related to mean daily dose of acenocoumarol (P = 0.41) [Tables 2 and 3]. On multivariate analysis, INR stability was related to the use of concomitant digoxin, PPI, and amiodarone and the presence of associated diseases, i.e., hypertension and diabetes mellitus [Table 4]. VKORC1 genotype was not related to the stability of INR control in both the univariate and multivariate analyses [Tables 3 and 4]. VKORC1 1639G>A GG phenotype was present in 127 patients (80.9%), GA genotype in 22 patients. (14%), and AA genotype in 8 patients (5.1%). Allelic frequency for G allele was 276 (87.9%) and A allele was 38 (12.1%) [Table 1].
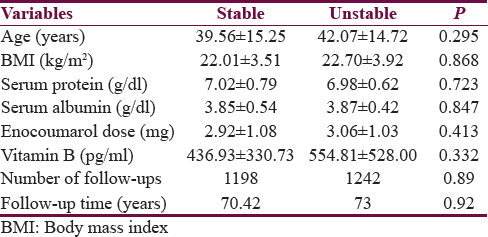
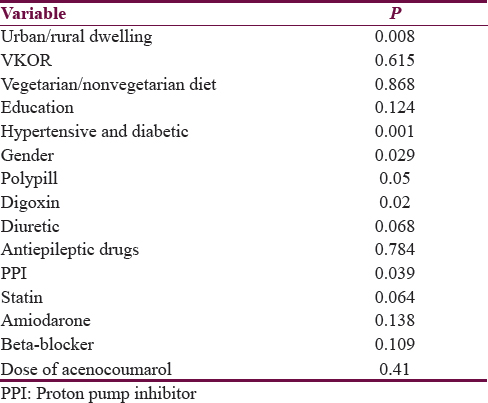
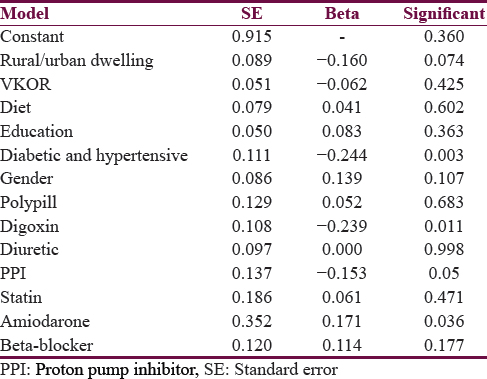
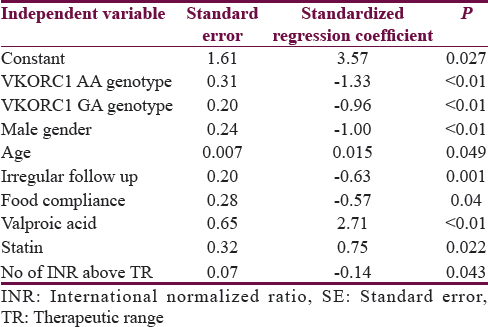
Predictors determining mean daily dose of acitrom acenocoumarol were age, male gender, GA and AA genotypes, alcohol, irregular follow-up, food compliance, and use of valproic acid and statins [Table 5].
DISCUSSION
In the present study, 49% of INR reports were in therapeutic range. This is in agreement with the reported frequency of 43%–72%.[411] The INR was in the therapeutic range of 51% in a retrospective USA study and 58% in a retrospective Canadian study.[11] In SPAF-II trial, 72% INR were in the therapeutic range.[12] In a study on anticoagulation in neurological patients, the quality of INR control in a group of 77 patients revealed that 39.3% of INR values were in the recommended range and only 42.9% patients had stable INR.[13] However, poorer quality of anticoagulation in our study may be due to physician's apprehension in using OAC due to risk of complications. Other factors may be due to infrequent INR testing as patients from remote or rural areas may have difficulty in reaching hospital because of economic or logistic reasons. Several studies have reported that optimal INR may be maximized by frequent INR testing. Optimal INR can be achieved in 90% on alternate day monitoring as compared to 50% when monitored monthly.[14]
In our study, retrospective data yielded inferior results compared to prospective data which may be due to frequent follow-up in prospective cases. We were able to get stable INR in 47.8% patients, which is better than some earlier studies.[15]
In our study, 89 patients (56.7%) out of 157 patients were vegetarian. However, the stability of INR was not related to vegetarianism (56% in stable vs. 57.3% in unstable). Dietary modification helped in stabilizing INR in 12 patients and drug modification in three patients in which valproic acid was replaced by levetiracetam. Diet plays an important role in maintaining stability of anticoagulation. Indians have a great diversity in diet with more than half of the population being vegetarian. Importance of diet relies not on avoiding diet rich in Vitamin K but on maintaining it in moderate constant level, thus avoiding loss of nutrition from them and maintaining stable INR at the same time. Vegetarian diet has variability in Vitamin K content which hinders the maintenance of stability of INR. The first-generation antiepileptic drug and other hepatic enzyme-inducing drugs have interactions with OAC and should be avoided.[161718] VKORC1 genetic polymorphism revealed GG genotype frequency in 127 patients (80.9%), GA genotype in 22 patients (14%), and AA genotype in eight patients (5.1%). Allelic frequency for G allele was 87.9% and A allele was 12.1%. This is slightly different from other studies from India. In a study from South India, Sivalingam et al. found GG genotype frequency to be 79.9%, GA to be 19.4%, and AA to be 0.7%.[19] They noted G allele frequency in 89.6% and A allele frequency in 10.6% of patients. Two studies on Indian population have reported the GG, AG, and AA genotype frequencies of VKORC1-1639G >A polymorphism as 80%, 13%, 7% and 88.3%, 9%, 2.7%, respectively[2021] Small study sample size may have contributed to this slight difference. In our study, female gender, rural dwelling, presence of diabetes and hypertension, use of multiple drugs such as digoxin, PPIs, and amiodarone were related to unstable INR, which is consistent with earlier reports.[11162223] Genotype was not related to the stability of INR. In a review of anticoagulation control in a cohort of over 100,000 patients, intake of 16 or more medications resulted in a 5% reduction in INR control compared with seven or fewer medications.[22] In our study, we found that 64 patients received >4 drugs out of a total of 157 patients. Thirty-nine patients (47.56%) in unstable INR group and 25 patients (33.33%) in stable INR group received ≥4 drugs. Reducing the number of medications prescribed may be helpful. A population-based cohort study evaluated the relationship between excessive anticoagulation and use of a PPI in 2755 outpatients receiving acenocoumarol.[23] Of these, 887 (32%) developed an INR >6.0. In our study, 14 patients were taking pantoprazole. Only 3 out of 14 had stable INR. PPI should be used cautiously and more frequent follow-up for INR monitoring may be required. We also had large population of patients with prosthetic heart valve (19 patients) who required higher level of anticoagulation which is more difficult to maintain.
Drug response was also variable. Six patients required very small dose (1 mg) and seven patients required extremely high dose (5 mg). Patient who required high dose had wild genotype of VKORC1 and patients who required small dose had minor allele genotype. Mean dose of acenocoumarol required in a patient was related to genotype, alcoholism, male gender, age, irregular follow-up, food compliance, and use of valproic acid and statins, which is in agreement with other studies.[16242526] A study from South India showed that mean daily warfarin dose is higher in patients with wild-type genotypes of CYP2C9 and VKORC1 compared to those with variant genotypes. Age, body mass index (BMI), duration of therapy, and genetic polymorphisms of CYP2C9 and VKORC1 together contribute to 36.1% variability in mean daily warfarin dose.[19] A study from North India showed that cytochrome P450 2C9 (CYP2C9) and Vitamin K epoxide reductase complex subunit 1 (VKORC1) polymorphisms are closely associated with maintenance dose of OACs.[20] In our study, BMI was not related to dose requirement. Mean dose of OAC required in wild allele (3.68 ± 0.87 mg) was higher as compared with that of minor allele (2.27 ± 0.67 mg). Mean dose requirement is dependent on other genes encoding for CYP2C9, CYP4F4, and others, which was not done in this study. We have studied VKORC1 genetic polymorphism which is the most important gene among others. We have not studied other genes which might have provided additional information. These genes should be studied on a larger scale to improve our understanding of the subject. This is a study based on a small sample size and from a single center. Therefore, the results cannot be extrapolated to community in general. Some of the results have been based on the outside results which may affect the quality of results although we have tried to verify the results from our laboratory. Other factors which may have been missed are intake of over-the-counter drugs such as pain killers, antipyretics, vitamins, calcium supplements, or other alternative medicines which may have effect on maintaining stability and dose requirement. Strength of the study lies in its predominant prospective design and clinical correlation of data which helped us in getting good follow-up from most of the patients which helped in fulfillment of the objective of the study. There are very few studies on neurological patients on this subject. Our study may contribute to the knowledge in this subject. More genetic studies on multiethnic society may identify additional genetic polymorphisms responsible for dosage variations among individuals. Future studies are also required to find rapid and cost-effective means to determine genetic polymorphisms to facilitate dose management prior to the initiation of therapy.
CONCLUSION
This study revealed stablily of INR following acenocoumarol in 47.8% patients VKORC1 gene polymorphism does not correlate with stability of INR but was related to the daily dose of OAC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is already known about this subject?
Vitamin K antagonists are drugs which have a narrow therapeutic index which is attributed to clinical as well as genetic variables. Various genetic polymorphisms are associated with dose requirement of OAC.
What this study adds?
It is less known whether genetic polymorphism is associated with stability of INR. The present study evaluates if genetic polymorphism is related to dose of VKA and stability of INR and to know predictors of stability of INR control.
Acknowledgment
We thanks Mr. Shakti Kumar for secretarial help.
REFERENCES
- Pharmacology and management of the Vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:160S-98S.
- [Google Scholar]
- Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019-26.
- [Google Scholar]
- EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229-35.
- [Google Scholar]
- Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: A systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:84-91.
- [Google Scholar]
- Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285-93.
- [Google Scholar]
- Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326-31.
- [Google Scholar]
- The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784-92.
- [Google Scholar]
- Analysis of factors affecting time in therapeutic range control after warfarin administration. Pharmazie. 2015;70:494-8.
- [Google Scholar]
- A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
- [Google Scholar]
- The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood. 2005;106:2329-33.
- [Google Scholar]
- Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: The international study of anticoagulation management (ISAM) J Thromb Thrombolysis. 2007;23:83-91.
- [Google Scholar]
- Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke prevention in atrial fibrillation II study. Lancet. 1994;343:687-91.
- [Google Scholar]
- Quality of anticoagulation therapy in neurological patients in a tertiary care hospital in North India. Indian J Med Res. 2016;143:428-33.
- [Google Scholar]
- A structured teaching and self-management program for patients receiving oral anticoagulation: A randomized controlled trial.Working group for the study of patient self-management of oral anticoagulation. JAMA. 1999;281:145-50.
- [Google Scholar]
- Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood. 2009;114:952-6.
- [Google Scholar]
- Drug interactions with warfarin: What clinicians need to know. CMAJ. 2007;177:369-71.
- [Google Scholar]
- Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med. 2010;123:151-7.
- [Google Scholar]
- Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-51.
- [Google Scholar]
- Effect of CYP2C9 and VKORC1 genetic polymorphisms on warfarin dose requirement in South Indian population. Indian J Physiol Pharmacol. 2013;57:308-17.
- [Google Scholar]
- Cytochrome P450 (CYP2C9*2,*3) & Vitamin-K epoxide reductase complex (VKORC1 -1639G&A) gene polymorphisms & their effect on acenocoumarol dose in patients with mechanical heart valve replacement. Indian J Med Res. 2013;137:203-9.
- [Google Scholar]
- Pharmacogenetic aspects of coumarinic oral anticoagulant therapies. Indian J Clin Biochem. 2011;26:222-9.
- [Google Scholar]
- Patient characteristics associated with oral anticoagulation control: Results of the Veterans AffaiRs Study to Improve Anticoagulation (VARIA) J Thromb Haemost. 2010;8:2182-91.
- [Google Scholar]
- Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol. 2011;153:379-85.
- [Google Scholar]
- Effect of VKORC1-1639 G and A polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients. Thromb Res. 2009;124:161-6.
- [Google Scholar]
- VKORC1 and CYP2C9 polymorphisms are associated with warfarin dose requirements in Turkish patients. Eur J Clin Pharmacol. 2008;64:889-94.
- [Google Scholar]
- Warfarin response and Vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clin Pharmacol Ther. 2007;81:742-7.
- [Google Scholar]






