Translate this page into:
The Profile of Amyotrophic Lateral Sclerosis in Natives of Western Himalayas: Hospital-Based Cohort Study
Address for correspondence: Dr. Sachin Sondhi, IGMC, Shimla - 171 001, Himachal Pradesh, India. E-mail: ssachin119@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Despite the disabling nature of amyotrophic lateral sclerosis (ALS), there are no contemporary data on clinical characteristics available from rural hilly states from India. Thus, the present study aimed at reporting clinical profile in ALS patients from natives of Western Himalayas.
Materials and Methods:
A total of 32 patients of ALS were enrolled over a period of 1 year (2013–2014) in the present study. The demographic profile, clinical characteristics, and risk factors were systematically recorded, and these patients were followed for 1 year.
Results:
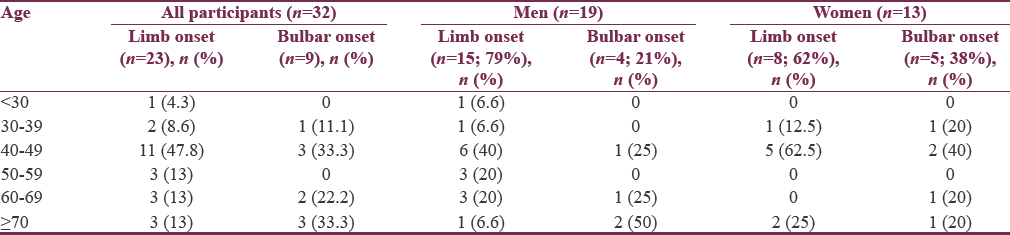
The mean age of ALS patients was 53 ± 15.88 (23–90 years). Maximum number of patients of both limb onset and bulbar onset were in the age group of 40–49 years [Figure 1]. Male to female ratio was 1.46. Limb-onset was seen in 23 (72%) and bulbar-onset in 9 (28%) of patients. Bulbar-onset was more common in females as compared to males. Mean duration of symptoms were 19.06 ± 24 months (range 4–120 months). None of the studied risk factor showed statistically significant association with outcome of the disease. No familial association was found. The most common site of weakness was upper limb distal weakness. Definite ALS was seen in 13 (40.6%) patients. Mean ALS functional rating scale (ALSFRS) at presentation is 35.7 ± 7.9. All patients were started on riluzole. Mean ALSFRS at 9-month follow-up was 32.9 ± 7.4. After 1 year of follow up, 5 out of 32 patients died and among them, 4 were of limb onset and 1 was of bulbar onset ALS. Mean age at death in males was 66 ± 16 years and in females was 56.33 ± 24.8 years; mean survival in these patients was 25 months.
Conclusion:
This present study highlights following findings: (1) Male preponderance is less common in our patients as compared to earlier reports from India. Bulbar onset is more common in elderly (age >60 years) females. (2) As per previous reports from India, when compared to Western population present study supports the fact of the younger age of onset and longer duration of symptoms and slow course of disease in Indian patients.
Keywords
Amyotrophic lateral sclerosis
Lou Gehrig's disease
Motor neuron disease
INTRODUCTION
Motor neuron disease (MND) is neurodegenerative disorder involving primarily motor neurons of cerebral cortex, brainstem, and spinal cord. MND is characterized by the gradual death of upper and lower motor neurons leads to loss of motor function and clinical syndrome of muscle weakness, wasting, and paralysis resulting in death typically within 2–3 years.[123]
Briefly, the presentations include amyotrophic lateral sclerosis (ALS), in which both the lower motor neurons and upper motor neurons are affected. It is frequently referred to as “Lou Gehrig's disease” in memory of the famous baseball player who died of ALS[4] in 1941. Other forms primary lateral sclerosis, which is less common than other presentation with only upper motor neuron involvement; and progressive muscular atrophy, with only lower motor neuron signs. Population-based studies have established that the incidence of ALS in Europe is fairly uniform at 2.16/100,000 person-years.[5] Although ALS affects people worldwide, an exact incidence of this disease is not yet known. Men have a higher incidence of disease (3.0/100,000 person-years; 95% confidence interval [CI] 2.8–3.3) than do women (2.4/100,000 person-years; 95% CI 2.2–2.6).
Genetic, environmental, occupational, toxins, high-level physical activity, electrical injury, physical trauma, medical illness, and high magnetic field exposed activity; all have been suggested by different investigators as causal factors at different times but not proved yet with certainty.[678910] The etiology of the disease is unknown. Riluzole, a glutamate antagonist, is the only drug approved by the Food and Drug Administration for the treatment of ALS. In two therapeutic trials, riluzole prolonged survival by 3–6 months.[1112]
Himachal Pradesh is located between 30“22’ and 30”12’ north latitude and between 75“47’ and 79”4’ east longitude. It is a mountainous state in northern India, situated in Western part of outer Himalaya in with altitudes ranging from 350 to 7000 meters above mean sea level. It is one of the least urbanized states in India, with predominantly agricultural economy. The lifestyle of population in the area differs from those living in plains. The contemporary data of clinical profile in patients of ALS are limited despite being disabling nature of disease, and also data profile of ALS patients from this region is not available. Thus, this study is planned to know the profile of ALS from the natives of this region.
MATERIALS AND METHODS
Study design
This is a single center tertiary care hospital-based prospective cohort study. This study was carried out from June 2013 to May 2014 in the Indira Gandhi Medical College (IGMC) and Hospital, Shimla (Himachal Pradesh). The study protocol was approved by the Institutional Ethical Committee of IGMC, Shimla. In the present study, we have analyzed clinical profile of ALS patients.
Study population
All consecutive patients with ALS visiting IGMC hospital between the year 2013 and 2014 were screened for enrolment in the study. Patients with ALS were diagnosed using standard El Escorial criteria. Informed consent was obtained from each eligible patient of ALS before recording the data.
Data collection
The data related to sociodemographic profile, medical history, risk factors, neurological examination parameters, and treatment practices were collected systematically at the time of enrolment in the study using predesigned structured data recording format. The electromyography (EMG), nerve conduction study (NCS), and magnetic resonance imaging (MRI) were done in every patient.
The further information was obtained as following
-
Age of onset, progression of symptoms, family history of disease and initial symptoms: the presence of fasciculation's, dysphagia, dysarthria, cramps, muscular weakness, and emotional lability
-
Localization of disease onset (bulbar vs. limb onset ALS)
-
ALS risk factor questionnaire was obtained by personal interview to obtain self-reported lifetime residential and occupational history and personal lifestyle factors. Risk factors studied were as follows (a) History of trauma requiring admission to hospital, (b) Family history of MND, (c) History of electric Shock, (d) History of smoking cigarettes, (e) History of living >12 months in a rural area, (f) History of farm work for >12 months, (g) History of possible occupational or hobby exposure to lead. (Construction batteries, Automobile industries, paints, as soldering materials in electronics), (h) History of pesticide or herbicide use.
All cases were classified according to El Escoreal criteria into definite, probable and possible ALS. ALS functional rating scale (ALSFRS) score calculated at the time of presentation and after 1 year of follow up.
Statistical analysis
The descriptive statistics of the study population were reported as counts and percentages for categorical variables and mean ± standard deviation for continuous variables with normal distribution. The gender-based distribution of sociodemographic parameters and clinical characteristics were compared using Chi-square test. The data analysis was done using STATA 13 statistical software (StataCorp, College Station, TX, USA). Statistical significance was defined as two-sided P < 0.05.
RESULTS
Demographic presentation
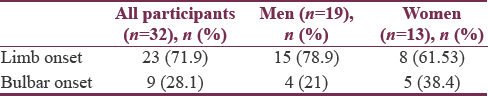
A total of 32 patients of ALS were enrolled over a 1 year period. In our study, mean age was a 53 ± 15.88 year with range from 23 to 90 years. Maximum number of patients; 15 (46.8%) were in the age group of 40–49 years [Table 1]. Numbers of male patients were 19 (59.3%) and of female patients were 13 (40.6%) [Figure 2]. Male to female ratio was 1.46:1. Maximum number of cases 25 (78%) were from rural background and remaining 7 (22%) are from urban background.


- Age-wise distribution according to onset of motor neuron disease

- Distribution of males and females in the study group
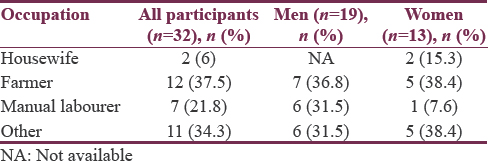
Most of the patients in our study were farmers 12 (37.5%) [Table 2]. Occupation of other patients were as follows (1 was carpenter, 2 were in army, 1 was doing automobile repair, 1 was driver, 1 was Mechanical worker in the form of electrical tandoor and boiler maker, 1 was Mason, 2 were peons in school, 1 was teacher, and 1 was in electricity board), and manual laborer were 7 (21.8%).
Risk factors
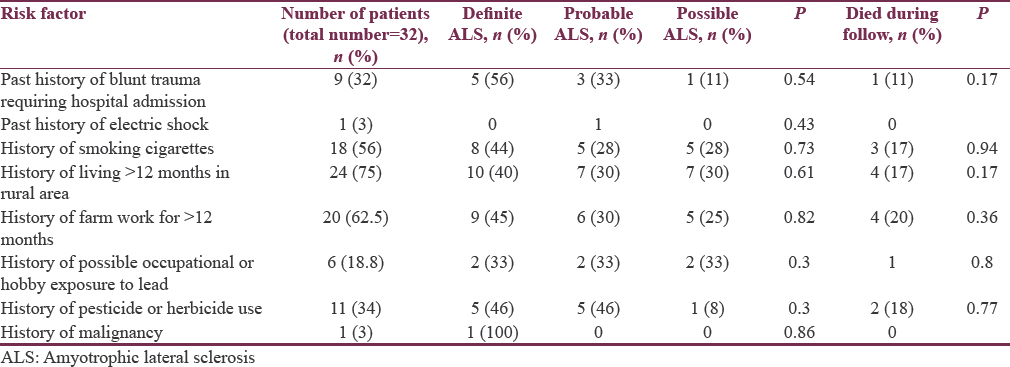
Risk factor distribution is described in Table 3. None of the studied risk factors showed statistically significant association with outcome of the disease.
Onset
Twenty-three (72%) patients had limb onset MND and 9 (28%) patients were having bulbar onset MND [Table 4]. Maximum number of patients of both limb onset and bulbar onset were in the age group of 40–49 years [Figure 1]. The 38% of females were having bulbar onset disease and 21% males having bulbar onset disease [Table 5].
Presenting complaint
Weakness in limbs was chief complaint 17 (53.12%) of patients followed by slurring of speech and difficulty in swallowing 8 (25%) and both weakness in limbs and slurring of speech in 7 (21.8%) patients, respectively.
Duration of symptoms in months
In our study, mean duration of symptoms were 19.06 ± 24 months with range from 4 to 120 months. Maximum of patients 12 (37.5%) presented with disease duration <12 months followed by patients 9 (28%) had duration <18 months. Only 2 (6.2%) patients had disease duration more than 8 years.
On neurological examination
Maximum number of patients 12 (37.5%) had atrophy of small muscles of hand followed by atrophy in bulbar muscles + upper limb in 12 (37.5%), upper limb in 3 (9.4%), upper limb and lower limb in 3 (9.4%), bulbar in 3 (9.4%) followed by 2 (6.2%) patients having no atrophy [Figures 3-5]. Fasciculations were present in 28 (88%) patients and Pseudobulbar affect was in 17 (53%) patients. Out of 32 patients, 17 (53.2%) had normal speech and dysarthria was seen in 15 (46.8%) of patients.

- Sites of atrophy
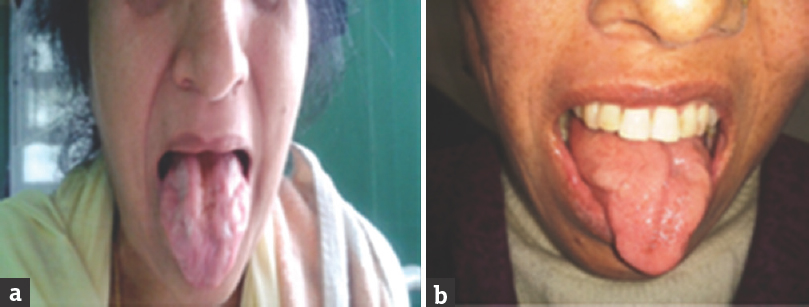
- (a and b) Both patients were having bulbar-onset motor neuron disease showing atrophy of the tongue
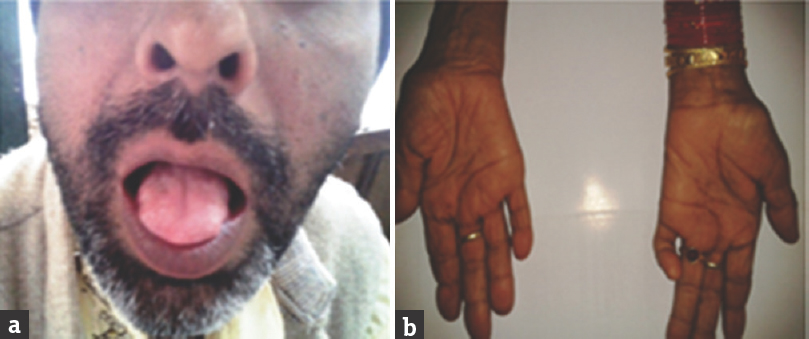
- (a and b) Bulbar onset motor neuron disease showing spastic tongue, second patient showing atrophy of small muscles of hand.
Diagnosis of amyotrophic lateral sclerosis according to El Escorial revised criteria
Out of 32 cases, definite ALS was seen in 13 (40.6%), probable ALS in 1 (3.1%), probable ALS laboratory supported in 10 (31.3%) and possible ALS in 8 (25%). Maximum number of males 9 (47.4%) had probable ALS and maximum number of females 8 (61.5%) had definite ALS.
Amyotrophic lateral sclerosis functional rating scale score at presentation
In our study, mean ALSFRS score at presentation was 35.7 ± 7.9 with range from 17 to 44. Males were having mean score of 37.2 ± 6.7 and females were having mean score of 31.7 ± 8.3. Bulbar onset had mean ALSFRS score of 39.2 ± 4.49 and limb onset had mean ALSFRS score of 36.8 ± 6.68.
At 9-month follow-up
After 1 year of follow up, 5 (15.6%) out of the 32 patients died and among them, 3 (60%) were female and 2 (40%) were male. The 3 (9.3%) of patients lost to follow up and remaining 24 (75%) of the patients had mean ALSFRS of 32.9 ± 7.4. PEG (percutaneous endoscopic gastrostomy) was done in one patient for progressive bulbar muscle weakness and dysphagia. In our study group, mean age at the time of death was 60.4 ± 22.7 years with range from 32 to 90 years. Mean age of death in males was 66 ± 16 years and in females were 56.33 ± 24.8 years. In our study, 2 (6.3%) patients had survival of more than 5 years. Out of five patients who died in study group limb onset of disease was present in 4 (80%) patients and bulbar onset was present in 1 (20%) patient. In remaining patients disease progression was slow. Out of 5 patients who died, 3 (60%) patients had respiratory involvement due to disease process itself; in remaining 2 (40%) cause of death was not known.
Investigations
After biochemical analysis 2 (6.2%) patients were found to have hypothyroidism, 2 (6.2%) patients were having leiomyoma, 1 (3.1%) patient was having carcinoma stomach.
EMG and NCS were done in all patients, and three sites were tested. Maximum of patients 31 (97%) had neurogenic pattern in EMG. Maximum number of patients 23 (72%) had normal NCS followed by 9 (28%) patients had pure motor axonal neuropathy.
Out of 32 patients, 19 patients underwent routine MRI brain/spinal cord and 13 patients underwent MRI + magnetic resonance spectroscopy (MRS), out of 19 patients who underwent routine MRI, 14 (73%) had normal brain MRI. Age-related atrophy in 5 (26.3%) patients. MRI cervical spine was done in patients having limb involvement, to rule out cord compression as a cause of weakness, none of the patients had cord compression. Some patients had degenerative changes in cervical spine. Thirteen patients underwent both MRI + MRS out of them 8 (61.5%) patients was having neurodegenerative changes in the brain with decrease in N-acetylaspartate/Cho ratio in motor cortex.
DISCUSSION
The present study from a tertiary care hospital provides the data regarding the clinical characteristics, complications with their risk determinants, and treatment practices among 32 ALS patients in the sub-Himalayan state of Himachal Pradesh in Northern India.
Demographic profile
Total numbers of patients in the study were 32. Numbers of male patients were 19 (59.3%) and of female patients were 13 (40.6%). Male to female ratio was 1.46:1. Majority of patients 15 (46.8%) were in the age group of 40–49 years with age of patients ranging from 23 to 90 years. In our study, mean age was 53 ± 15.88 years (23–90). Twenty-three (72%) patients were having limb onset and 9 (28%) were having bulbar onset.
Comparison with studies from India
In a study from Eastern India on ALS, limb onset was seen in 43.7% and bulbar onset in 33.3%. The M:F ratio was 4.3:1, with maximum number of patients between 41 and 50 years of age.[13] A study from Northern India with 62 cases of ALS showed a M:F ratio of 4.6:1, mean age of onset of 46.9 years, limb onset in 70.9%, bulbar onset in the remaining.[14] Another study, Rai and Jolly reported a M:F ratio of 6:1 among their 70 cases with ALS which was significantly high as compared to our series.[15] In yet another study from West India 82 cases of ALS had a M:F ratio of 2:1 and a high proportion had onset before the age of 40 year.[16]
Nalini et al. had shown male predominance (M:F 3:1) and mean age of 46.2 ± 14.1 years (range 18–85).
These findings are also similar to our series except for the sex ratio. Our study when showed M:F ratio 1.4:1. Male preponderance is less common in our study when compared with earlier reports from India. This is due to multifactorial nature of disease as many factors such as genetic, environmental, and social-cultural practices are related to the development of ALS and these factors vary from region to region.
Comparison to Western literature
When compared to the Western series[31718192021] where the mean age of onset in most studies ranges from 55 to 65 years of age, the mean age of onset is about one to two decades earlier among our patients and also in the earlier reports from India.[2223242526141516]
In our study, among 32 patients, maximum number of cases 25 (78%) were from rural background as seen in a study conducted by Das et al.[27] Most of the patients in our study were farmers 12 (37.5%) followed by patients engaged in other activities were 11 (34%), and manual laborer was 7 (21.8%). A significant number of the ALS patients in the study were the rural domicile people and were farmers. It has been detected as a risk factor of ALS in several studies.[28] Rural people are exposed to several chemicals used in the agricultural fields land may have repeated physical trauma arising from their physical nature of jobs.
Risk factors
There were number of risk factors associated with the development of MND studied in various studies.[789] In our study, 56% of patients were smokers [Table 3]. Our findings are comparable to most of other studies. Probably, a myriad of toxins associated with smoking may play a role in causing or triggering ALS by inducing oxidative stress. Manual laborers in the study used to do work in the construction of buildings and roads this may probably play a role in triggering ALS. One (3.1%) patient had carcinoma stomach. After statistical analysis, none of risk factor is seen to be associated with severity of the disease.
Onset and duration of symptoms
Twenty-three (72%) patients were having limb onset and 9 (28%) were having bulbar onset. In our study, bulbar onset was slightly more common in females (38%) as compared to males (21%). Out of total 9 cases of bulbar onset, 5 (56%) patients were more than 60 years of age. Our results were similar to study “Assessment of Pulmonary Function in ALS” done by Talakad et al.[29] It appears that motor neurons supplying bulbar musculature are more resistant to the disease process when compared to other motor neurons.
Comparison of duration of illness with studies from Indian and Western population
Mean duration of symptoms was 19.06 ± 24 months with range from 4 to 120 months. Limb-onset had mean duration of symptoms 20 months and bulbar onset had mean duration of 14 months. Our results were similar to, Indian study done by Nalini et al.[22] that showed mean illness duration was 17.7 ± 20.7 months. The mean duration of illness from disease onset in our patients was 19 months similar to 17 months as reported by Norris et al.,[3] but higher as compared to 13.3 months in another Western study.[20] This longer duration of symptoms in patients may reflect the benign/relatively slow course of ALS in our population.
In our study, out of 32 patients, maximum number of patients 19 (60%) were having body mass index (BMI) <18 kg/m2. Similar findings were seen in study done by Doyle et al.[10] There was a statistically significant reduction in risk of MND with increasing body mass. As in our study, we did not have baseline BMI of patients before onset of disease. Hence, we could not draw any inference from our study.
Definite ALS was seen in 13 (40.6%), probable ALS 11 (34.3%), and possible ALS in 8 (25%). Our institute is a tertiary care center; and hence, patients come to us mostly on referral basis at a late stage of the disease course, and a diagnosis of definite ALS is made in many patients before they visit our institute. Out of 32 patients, 31 (97%) had neurogenic pattern in EMG similar to seen by Nalini et al.[22] in 96.5% of patients.
Amyotrophic lateral sclerosis functional rating scale score and 9-month follow-up
Females were having low ALSFRS score as compared to males (31.7 - females/37.2 - males), more number of deaths (3 - females/2 - males), and early mean age at the time of death (56.33 year-in females and 66 year-in males). From these parameters, it can be concluded that females have worse prognosis than males. Results are similar to study[30] “Prognosis in ALS” done in Washington in 180 patients of ALS, which found that, older age and female sex were strongly associated with poor survival. Out of five patients who died in study group limb onset of disease was present in four patients and bulbar onset was present in one patient.
Limitations
The small size of study participants and only 1 year of follow-up period are the potential limiting factors in our study.
CONCLUSION
This is the first hospital-based observational study from rural hilly areas of India and also the first study of the ALS patients from India in the 21st century. This present study highlights following findings:
-
Male preponderance is less common in our patients as compared to earlier reports from India. Bulbar onset is more common in elderly (age >60 years) females and females have worse prognosis than males
-
As per previous reports from India, when compared to Western population present study supports the fact of younger age of onset and longer duration of symptoms and slow course of disease in Indian patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Avoiding false positive diagnoses of motor neuron disease: Lessons from the Scottish motor neuron disease register. J Neurol Neurosurg Psychiatry. 1996;60:147-51.
- [Google Scholar]
- Terminology and classification of ALS. In: Amyotrophic Lateral Sclerosis: Contemporary Neurology Series 49. Philadelphia: FA Davis; 1998. p. :18-33.
- [Google Scholar]
- Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. 1993;118:48-55.
- [Google Scholar]
- Amyotrophic lateral sclerosis: Possible role of environmental influences. Neurol Clin. 2005;23:461-84.
- [Google Scholar]
- Case control studies of motor neuron disease: Association with mechanical injuries. Arch Neurol. 1981;38:220-6.
- [Google Scholar]
- Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:558-61.
- [Google Scholar]
- Environmental and occupational risk factors for amyotrophic lateral sclerosis: A case control study. Neurodegener Dis. 2014;14:31 8.
- [Google Scholar]
- Incidence of and risk factors for motor neurone disease in UK women: A prospective study. BMC Neurol. 2012;12:25.
- [Google Scholar]
- A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585-91.
- [Google Scholar]
- Dose ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425-31.
- [Google Scholar]
- Pattern of motor neurone disease in Eastern India. Acta Neurol Scand. 1997;96:14-21.
- [Google Scholar]
- Pattern of motor neuron disease in North India and wasted leg syndrome. In: Gourie-Devi M, ed. Motor neuron disease. New Delhi: Oxford and IBH; 1987. p. :147-63.
- [Google Scholar]
- Motor neuron disease in West India. In: Gourie Devi M, ed. Motor Neuron Disease. New Delhi: Oxford and IBH; 1987. p. :165-70.
- [Google Scholar]
- Patient resistance and prognosis in amyotrophic lateral sclerosis. Mayo Clin Proc. 1976;51:537-41.
- [Google Scholar]
- Survival of patients with amyotrophic lateral sclerosis in a population based registry. Neuroepidemiology. 2005;25:114-9.
- [Google Scholar]
- Assessment of pulmonary function in amyotrophic lateral sclerosis. Indian J Chest Dis Allied Sci. 2009;51:87-91.
- [Google Scholar]
- Amyotrophic lateral sclerosis in Olmsted county, Minnesota, 1925 to 1998. Neurology. 2002;59:280-2.
- [Google Scholar]
- Prognosis in amyotrophic lateral sclerosis: A population based study. Neurology. 2003;60:813-9.
- [Google Scholar]
- Pattern of motor neurone disease in the young in North India. J Assoc Physicians India. 1975;23:695-701.
- [Google Scholar]
- Madras pattern of motor neuron disease in South India. J Neurol Neurosurg Psychiatry. 1988;51:773-7.
- [Google Scholar]
- Biochemical aspects of motor neurone disease – Madras pattern. J Neurol Neurosurg Psychiatry. 1973;36:753-6.
- [Google Scholar]
- Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012;4:350-5.
- [Google Scholar]
- Epidemiology of ALS. In: Mitsumoto H, Przedborski S, Gordon PH, eds. Amyotrophic Lateral Sclerosis. New York: Taylor and Francis; 2006. p. :17-41.
- [Google Scholar]
- Factors predicting one year mortality in amyotrophic lateral sclerosis patients – Data from a population based registry. BMC Neurol. 2014;14:197.
- [Google Scholar]
- Clinical characteristics and survival pattern of 1,153 patients with amyotrophic lateral sclerosis: Experience over 30 years from India. J Neurol Sci. 2008;272:60-70.
- [Google Scholar]






