Translate this page into:
The entire spectrum of typical and atypical magnetic resonance imaging changes in a single case of Wilson's disease
Address for correspondence: Dr. Ujjawal Roy, Department of Neurology, Bangur Institute of Neurosciences, IPGMER, Kolkata, West Bengal, India. E-mail: drujjawalroy@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Magnetic resonance imaging (MRI) of brain plays a key role in supporting the diagnosis of Wilson's disease. Classical radiological findings such as symmetrical gray matter (basal ganglia and thalamus) hyperintensities on T2-weighted MRI sequence are well known. At the same time, atypical MRI signs such as “Face of the giant Panda” and “Trident Pons sign” have been reported in some cases. However, the complete spectrum of common along with atypical MRI signs is rarely reported in a single case in medical literature. This case displays the entire range of MRI changes in Wilson's disease.
A 23-year-old boy presented with history of dystonic posturing of his extremities for the past 2 years. He also had drooling of saliva and slurred speech for the same duration. There was no history of seizures or cognitive decline. The symptoms were insidious in onset and progressively worsened over the duration of illness. On general examination, the patient had facial muscles dystonia producing a characteristic “risus sardonicus” facies. Besides, he had severe hypokinetic dysarthria and was detected to be positive for “Kayser–Fleischer” (K-F) ring on torchlight which was further confirmed on slit lamp examination. Neurological examination revealed dystonic posturing of extremities along with cogwheel rigidity. Rest of the systemic examination did not reveal any abnormality. On investigating the case, patient's MRI of the brain showed symmetrical hyperintensities in basal ganglia and thalamus and asymmetrical subcortical white matter hyperintensities on T2-weighted imaging sequence [Figure 1a and b]. MRI was also remarkable in revealing classical changes in midbrain indicative of the “Face of giant panda” along with central pontine myelinolysis (CPM)-like changes in pons, appearing as a “Bisected pons” [Figure 1c and d]. Considering MRI features and K-F ring suggestive of Wilson's disease, patient's further investigations were directed accordingly. Serum ceruloplasmin level was low (3 mg/dL). 24-h urinary copper excretion was elevated (200 mcg/day). The patient was thus diagnosed as a case of Wilson's disease, and treatment was started with Zinc and Penicillamine therapy to which patient responded well in due course of treatment. His limbs and face dystonia improved significantly after 6 months follow-up.
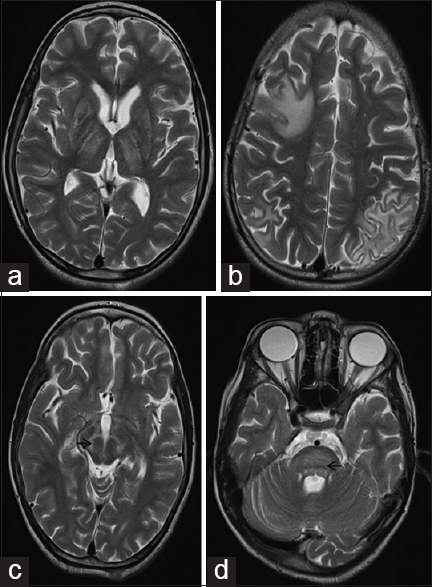
- T2-weighted magnetic resonance imaging brain shows bilateral symmetrical basal ganglia and thalamic hyperintensities (a) along with asymmetrical subcortical white matter hyperintensities. (b) Midbrain signal changes show the classical “Face of giant panda” sign with tegmentum involvement appearing hyperintense (arrow) while the relatively preserved red nucleus and lateral portion of substantia nigra pars reticulata, appearing hypointense. (c) Central pontine myelinolysis-like changes in the pons resulted in a “Bisected Pons” appearance with relatively preserved pontine tissue showing up as a dark horizontal line (arrow) (d)
Neurological manifestations of Wilson's disease are ascribed to abnormal deposition of copper in brain tissues, especially deep gray matter (basal ganglia and thalamus) and subcortical white matter. Brainstem is also involved though less commonly, but with radiological signs such as “Panda sign” and “CPM-like changes” with specific patterns, which are considered as defining for the illness. Bilateral symmetrical hyperintensities in lentiform nucleus, caudate, and thalamus are well known.[1] Brainstem changes occur with preferential involvement of midbrain. A retrospective study by Kim et al. reported midbrain signal changes in 40% of the study subjects.[2] However, classical midbrain sign of “Face of giant panda,” which is characterized by high T2-weighted signal intensity in tegmentum with relative preservation of red nucleus and lateral part of substantia nigra pars reticulata, is occasionally reported.[3] Pons is rarely involved in Wilson's disease. In the same study by Kim et al., Pontine changes were observed only in one case.[2] MRI signal changes in pons typically create CPM-like picture in Wilson's disease. Although reported singularly, these CPM-like changes in pons classically have three distinct patterns for Wilson's disease: (1) The most common pattern of round shape, (2) bisected, and (3) trisected or “trident” sign.[4] T2-weighted hyperintensities in subcortical white matter of frontal, parietal, and temporal lobes are also uncommonly reported.[5] Recapitulating, isolated MRI findings, typical as well as atypical, are often reported in different patients. Howbeit, the entire range of MRI features in one patient, have not been reported in medical literature. The present case is remarkable because it displays almost all the MRI characteristics of Wilson's disease, whether common or atypical, in a single patient.
Having on display the entire spectrum of MRI findings of Wilson's disease, this case may serve as an excellent learning source for reviewing the key radiological features of the disease for physicians and medical graduates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cranial MR in Wilson disease: Abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am J Neuroradiol. 1995;16:2021-7.
- [Google Scholar]
- MR imaging of the brain in Wilson disease of childhood: Findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol. 2006;27:1373-8.
- [Google Scholar]
- Central pontine signal changes in Wilson's disease: Distinct MRI morphology and sequential changes with de-coppering therapy. J Neuroimaging. 2007;17:286-91.
- [Google Scholar]
- Extensive gray and white matter abnormalities in Wilson's disease: A case report. Indian J Radiol Imaging. 2006;16:91.
- [Google Scholar]





