Translate this page into:
Synergy-Based Motor Therapy Inducing Favorable Changes in Motor Function Components among Poststroke Subjects: A Single-Group Study
Kamal Narayan Arya, PhD Department of Occupational Therapy, Pandit Deendayal Upadhyaya National Institute for Persons with Physical Disabilities New Delhi 110002 India kamalnarya@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Synergy is an outcome of multiple muscles acting in a synchronized pattern, controlled by the central nervous system. After brain insult, a set of deviated movement pattern emerges in the affected limb. The methods to train synchronization of muscles may diminish the deviated movement augmenting neuromotor control. The purpose of this investigation was to develop a synergy-based motor therapy (SBMT) protocol for the paretic upper limb in poststroke subjects. Further, the feasibility and effectiveness of the program was evaluated. .
Methods The design was Pretest–posttest single-group assessor-blinded trial. Department of occupational therapy of a national institute for persons with physical disabilities was the study site. There were 40 study subjects (23 men, ranging from 40 to 60 years, 18 subjects with hemorrhagic cerebrovascular accident, and > 6 months after the accident) exhibiting motor paresis of half side of the body. SBMT is a stage-specific regime based on the linkage between the deviated and usual muscle action. SBMT items were selected considering the strength and magnitude of the deviated motor components. The movement linkages were utilized to dissociate strong coupled components; for instance, forearm pronation-supination with elbow 90-degree flexion. Fugl-Meyer Assessment (upper extremity) (FMA-UE), Wolf Motor Function Test (WMFT), and Barthel Index (BI) were applied to quantify the motor status, motor functional ability of the upper extremity, and self-care activities, respectively.
Results All the enrolled subjects could perform their corresponding SBMT sessions. Posttreatment, FMA-UE improved significantly (p < 0.001) from mean of 26.30 (standard deviation [SD] 15.02) to 35.20 (SD 17.64). Similarly, the WMFT both time (in seconds) and quality also positively improved significantly (p < .001) from mean of 76.77 (SD 54.73) to 64.07 (SD 56.99) and 1.34 (SD 1.06) to 1.87 (SD 1.34), respectively. BI improved from 79.88 (SD 17.07) to 92.62 (SD 21.2) after the intervention (p < 0.001).
Conclusion SBMT protocol was a feasible and effective intervention to facilitate motor function components in chronic hemiparetic subjects. The regime could be considered as a potential intervention for stroke rehabilitation. Further trials and use of sophisticated measures are recommended to authenticate the outcome of this investigation.
Clinical Trial Registration Clinical Trial Registry of India as CTRI/2017/10/010162 on October 23, 2017 (retrospectively).
Keywords
CVA
muscle linkage
neuroplasticity
motor control
dissociation
Introduction
Half side motor weakness is the most challenging and unresolved issue after stroke. Upper extremity weakness is usually more severe and less recoverable than the lower extremity. Thus, there is a mismatch between the recovery and functional demand of the upper limb among poststroke survivors.1 Voluntary motor control gets hampered and takes a shape of stereotypical motor presentation. Poor motor control or inability to dissociate the joint movement should be a key target in the poststroke rehabilitation.2 The stroke subjects appear with flaccidity to severe spasticity and if the recovery proceeds they reach to a stage of usual motor performance. During the progression from flaccid to spastic stage, an individual exhibits abnormal deviated motor presentation of the upper extremity.3 Unfortunately, a large number of subjects gets stagnant at a severe spastic stage and less number of subjects progress and achieve good motor recovery.
The upper limb functions are outcome of synergistically linked movements depending upon a task.4 Synergy comprises actions of muscles coordinated by the nervous system to produce efficient functions.5 Such normal coordination gets hampered and appears as an abnormal muscle synergy among poststroke subjects.6 The upper limb exhibits abnormal coupling due to lack of motor control at individual joint. The deviated motor behavior usually leads to a typical upper limb pattern. The pattern comprises of adducted and medially rotated arm, flexed and pronated forearm, and flexed wrist and fingers.3 7
The synergistic motor behavior reflects the level and location of brain insult and predicts the prognosis.8 9 In response to cerebrovascular accident, the cortex exhibits abnormal developmental reflexes.10 The emergence of deviated movements will depend upon the severity of the abnormal reflexes.11 The damage results into incorrect timing of action sequence, loss of interjoint coordination, and ultimately abnormal movement pattern.12 The deviated movement pattern constrains the voluntary motor action.3 13 Thus, the primary goal of poststroke motor intervention should focus on enhancing the normal synergy and developing individual joint control.14
In stroke, various interventions have been developed for the upper limb impairment. However, only few motor therapies utilized the concept of synergy; for instance, the conventional approaches such as Brunnstrom motor therapy and Bobath technique.3 15 The motor synergy may be considered as a physiological marker for poststroke recovery and thus the patterns should be utilized to form the basis of the motor therapies in stroke.16 Brunnstrom movement therapy emphasizes on brain reflexes and associated reactions to develop limb synergies and spasticity. The therapy advances from mass stereotyped movements to the combined synergies and then to an individual joint movement. The approach also considers the use of the abnormal synergistic pattern in daily functions, if the subject gets stagnant at the mid recovery stage. The regime has been utilized in clinical practice; however, the evidence for the entire aspect of the approach is sparse. Bobath's approach focuses on abnormal tone, asymmetry, and posture. The technique emphasizes on reflex-inhibiting patterns and facilitating the girdle movements. Functional use of the affected side with use of adaptive equipment avoiding the movement compensation is also the part of this approach. This technique lacks stagewise motor training as well as specific methods for the individual joints of upper extremity. The Bobath's approach has not been found to be superior to the other motor therapies of stroke.17
Unquestionably, there is a strong relation between the motor components of the synergies. The achievements of challenging components are indicators of recovery in poststroke subjects. Conventionally, the issue has been considered in clinical practice; however, due to either lack of investigations or favorable evidence the developed techniques are not widely practiced. The various synergistic components have been studied and found to be related with the motor recovery.18 However, a synergy-based protocol for all the motor components across the stages has not been explored yet.
Based on the recent concept of synergy, synergy-based motor therapy (SBMT) investigation will lead to a formulation of an innovative motor regime. The program will be primarily directed to induce normal synergistic pattern by means of positioning, movements, and activities, across all the stages of stroke. Further, the protocol is planned to be well-structured considering each motor component of the paretic upper extremity. The investigation was based on the hypothesis that the synergy-based protocol would substantially enhance the motor recovery in stroke. Further, the objective was to determine the feasibility and effectiveness of SBMT on motor and functional abilities of the paretic arm and hand.
Methods
Design
The investigation was a single-group, pretest–posttest, assessor-blinded trial. Pre- and postassessments were performed by two independent evaluators.
Setting
The research was accomplished in the functional therapy unit (department of occupational therapy) of a national institute in the field of physical disabilities situated in a metropolitan city of northern India.
Participants
The recruited participants demonstrated the following conditions: (1) nontraumatic stroke, (2) ischemic or hemorrhagic type, (3) right or left paresis, (4) more than 6 months of poststroke duration, (5) Brunnstrom recovery stage (BRS) ≥ 2 and above for the arm, and (6) age under 60 years. The participants were not enrolled in the study on showing any of the following features: (1) receptive communication disorder, (2) painful shoulder, (3) subluxed shoulder, (4) contractures or deformities, (5) severe cognitive and perceptual deficit (as evaluated by the clinical tests: line bisection, copying and drawing, and functional performance related to time, place, and orientation), and (5) cardiovascular instability.
Ethical Approval
The investigation was endorsed by the institutional ethics committee (PDUIPH/IEC/V/2014/01) of the research site. The recruited subjects were provided a comprehensive informed consent form about the study, which they signed. The study protocol was explained to the enrolled subjects and their family members before getting the duly filled informed consent form.
Sessions
After screening, a detail clinical assessment and preintervention scoring using outcome measures was performed for each subject. Two sessions were devoted for the evaluation. Forty-eight intervention sessions (3/week) were provided during the period of 4 months. The duration of first 24 sessions was 1 hour (30 minutes conventional + 30 minutes SBMT protocol) and the next 24 sessions was 1.5 hours (30 minutes conventional + 60 minutes SBMT protocol). The activities were performed for 5 to 10 repetitions, ranging from 1 to 3 sets. The subjects were allowed to take rest for the period of 2 minutes after every 15 minutes of therapy for the first 24 sessions. The period was reduced to 1 minute for the next 24 sessions.
Experimental Intervention
The intervention protocol is based on synergistic movement patterns available in normal individuals and deviated in stroke patients. Poststroke, synergistic movements get distorted in form of awkward and abnormally coupled motor behavior.19 The purpose of SBMT regime was to facilitate efficient and coordinated motor functions. Considering the outcome of the previous investigation,20 a linkage framework for training a specific motor component was developed. The key principles of SBMT are (1) normal synergistic movements are fundamental elements of a functional task, (2) poststroke motor recovery occurs in a hierarchical sequential pattern; reflexive to synergy to out-of-synergy movements, (3) proximal joint control influence the distal joint movement, (4) distal motor control enhances when practiced simultaneously with specific proximal movement, (5) normal muscle synergy is utilized to dissociate the abnormal synergy, (6) motor training varied from reflexive, assistive, guided, to active movements, and (7) BRS of arm and hand and Fugl-Meyer Assessment (FMA), upper extremity section are considered as a framework for stage-specific treatment protocol. The SBMT protocol was imparted by the authors (S.P. and K.N.A.), occupational therapy practitioners, who had been working in the field of stroke rehabilitation for two decades. The intervention was provided on individual basis to each enrolled subject.
The key principles for SBMT are shown in Supplementary Table S1 (available in the online version only) whereas the detailed protocol is provided in the Supplementary Table S2 (available in the online version only) and Fig. 1. The (TIDieRTemplate for Intervention Description and Replication) Checklist is provided in Supplementary Table S3 (available in the online version only).

-
Fig. 1 (A–E) Exhibiting the subjects performing activities of the synergy-based motor therapy (SBMT) protocol. (A) Shoulder abduction with external rotation. (B) Elbow extension with wrist dorsiflexion. (C) Elbow extension with wrist circumduction. (D) Forearm supination pronation at elbow 90 degrees. (E) Shoulder internal external rotation with elbow neutral.
Fig. 1 (A–E) Exhibiting the subjects performing activities of the synergy-based motor therapy (SBMT) protocol. (A) Shoulder abduction with external rotation. (B) Elbow extension with wrist dorsiflexion. (C) Elbow extension with wrist circumduction. (D) Forearm supination pronation at elbow 90 degrees. (E) Shoulder internal external rotation with elbow neutral.
Outcome Measures
To assess the feasibility of SBMT, the following aspects were examined: (1) number of sessions attended by the subjects, (2) safety (such as fatigue, pain, muscle soreness, discomfort, and fall), and (3) ability to follow the procedures of intervention.
Primary
Brunnstrom Recovery Stage
Signe Brunnstrom classified motor recovery of arm and hand in six stages. BRS, ordered into 7 level varied from absent motor control (flaccid stage) to usual motor performance of individual movement (close to normal movement). Subject is being instructed to perform a set of prescribed movements at a particular stage.
BRS is commonly used in stroke rehabilitation-related studies.21 22 It has exhibited adequate validity and excellent responsiveness.23 24 25
Fugl-Meyer Assessment
FMA determines the status of the motor control of paretic limb. The motor components are categorized and assessed as per the sequential recovery in stroke subjects. The assessor records the performance based on the motor execution of the upper extremity.26 Comprehensively, 33 items of upper extremity (FMA-UE) subsection are ranked from deviated to under control motor pattern. In view of the ability to perform, the items are awarded a score of 0 (no movement) or 1 (partial movement) or 2 (full movement).
FMA exhibited adequate reliability and validity.27 28 29 The minimum clinically important difference (MCID) for FMA-UE is recorded as 4.25 to 7.25 for chronic stroke subjects.30
Wolf Motor Function Test
Wolf Motor Function Test (WMFT) examines the motor and functional ability of upper limb among stroke population. The scale comprises of total 17 activities performed over a standardized template pasted on a table. The time and the quality of various listed activities are measured on a 6-point ordinal scale. WFMT shows excellent reliability and internal consistency for both time and quality of performance.31 32 MCID has been estimated as 1.5 to 2 seconds (time) and 0.2 to 0.4 (quality).33
Secondary
Barthel Index
Barthel Index (BI) comprises 10 functional activities, evaluating self-care tasks. Every item quantified separately with a score of 0, 5, 10, or 15. The maximum score is 100, indicating the full independent performance. The evaluated items are feeding, wheelchair and bed transfers, toilet activity and personal hygiene, mobility, locomotion, ascending and descending stairs, and bladder-bowl control. It is a commonly used functional assessment tool for stroke subjects.34 35 BI exhibited adequate reliability and validity.36 37
Statistical Analysis
Being a single-group design, the paired t-test was applied to determine the change between the pre- and postintervention scores of the assessment tools. The findings were considered significant for p < 0.05. The prescores were treated as postscores for the subjects who were lost to follow-up. IBM SPSS version 23 was utilized to perform the statistical analysis.
Results
The flow of the participants during the study is provided in Fig. 2. Forty poststroke hemiparetic participants were enrolled for SBMT investigation. There were 23 males, 22 ischemic and 20 were right hemiparetic subjects. The average age duration of the study participants was 50 years and their poststroke duration was 18 months. The clinical profile and the distribution (as per BRS [arm]) of the study participants are provided in Tables 1 and 2, respectively.
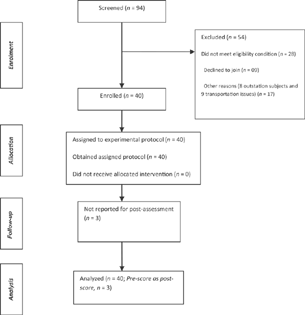
-
Fig. 2 Flowchart of study.
Fig. 2 Flowchart of study.
|
Clinical profile |
N = 40 |
|---|---|
|
Age in years (mean ± SD) |
50.25 (12.84) |
|
Men/Women |
23 (57.5%)/17(42.5%) |
|
Duration of onset in months (mean ± SD) |
17.75 (9.49) |
|
Right/Left paresis |
20 (50%)/20(50%) |
|
Ischemic/Hemorrhagic |
22/18 (55%/45%) |
|
Frontoparietal/Basal ganglia/Thalamic-internal capsule/Multiple/Others |
05 (12.5%)/09 (22.5%)/02 (5%)/13 (32.5%)/11 (27.5%) |
|
Risk factors |
|
|
Obesity |
06 (15%) |
|
Alcoholic |
11 (27.5%) |
|
Smoking |
5 (12.5%) |
|
Hereditary |
5 (12.5%) |
|
Hypertension |
36 (90%) |
|
Diabetes |
16 (40%) |
Abbreviation: SD, standard deviation.
|
Brunnstrom's recovery stage (BRS) of arm |
Number of participants (n = 40) |
|
|---|---|---|
|
Preintervention |
Postintervention |
|
|
BRS 2 |
13 (32.5%) |
8 (20%) |
|
BRS 2–3 |
17 (42.5%) |
12 (30%) |
|
BRS 3 |
06 (15%) |
10 (25%) |
|
BRS 3–4 |
03 (7.5%) |
05 (12.5%) |
|
BRS 4 |
01 (2.5%) |
04 (10%) |
|
BRS 4–5 |
00 (0%) |
01 (2.5%) |
SBMT protocol was found to be feasible for the poststroke hemiparetic subjects. The study participants could perform the stage-appropriate activities of the intervention. None of the subjects reported any adverse event during the protocol. All the participants attended the planned 48 intervention sessions, except two subjects who missed one and two sessions, respectively, due to their personal reasons.
Table 2 also shows the change in distribution of subjects according to the BRS-A after the intervention. Further, 6 (15%), 19 (47.5%), and 9 (22.5%) subjects were recorded at BRS-H 1, 2, and 3, respectively, preintervention. Posttreatment, there were 3 (7.5%), 14 (35%), and 7 (17.5%) subjects at BRS-H 1, 2, and 3, respectively. Six subjects were at BRS-H 4 prior to the protocol, after intervention eight subjects achieved the same stage. None of the subjects could achieve BRS-H 5 and 6.
Posttreatment, the motor outcome measures (FMA-UE) improved significantly (p < 0.001) from mean of 26.30 (standard deviation [SD] 15.02) to 35.20 (SD 17.64). The WMFT both time and quality also positively improved significantly (p < 0.001) from mean of 76.77 (SD 54.73) to 64.07 (SD 56.99) and 1.34 (SD 1.06) to 1.87 (SD 1.34), respectively. The BI changed significantly (p < 0.001) from mean of 79.88 (SD 17.07) to 92.62 (SD 21.24). Table 3 shows the detailed inferential statistics for the changes on all the outcome measures.
|
Outcome |
Preintervention score (mean [SD]) |
Postintervention score (mean [SD]) |
95% CI |
p-Value |
|---|---|---|---|---|
|
FMA-UE |
26.30 (15.02) |
35.20 (17.64) |
2.37–2.79 |
< 0.001 |
|
FMA-UA |
18.88 (7.77) |
23.90 (8.28) |
3.63–6.4 |
< 0.001 |
|
FMA-WH |
7.42 (8.41) |
11.30 (9.88) |
2.37–5.37 |
< 0.001 |
|
WMFT-Time |
76.77 (54.73) |
64.07 (56.99) |
9.01–8.65 |
< 0.001 |
|
WMFT-Quality |
1.34 (1.06) |
1.87 (1.34) |
0.36–0.68 |
< 0.001 |
|
BI-total |
79.88 (17.07) |
92.62 (21.2) |
5.86–19.63 |
< 0.001 |
Abbreviations: BI, Barthel Index; CI, confidence interval; FMA, Fugl-Meyer Assessment; SD, standard deviation; UA, upper arm; UE, upper extremity; WH, wrist-hand; WMFT, Wolf Motor Function Test.
The analysis for the right and left hemiparetic subgroups also exhibited significant changes (p < 0.001) after the SBMT regime. The right subgroup improved on FMA-UE from mean of 27.9 (SD 15) to 38.35 (SD 18.91) while the corresponding left subgroup also recovered from mean of 24.70 (SD 15.25) to 32.05 (SD 16.14). Similarly, on WMFT-time the right subgroup reduced to mean of 57.38 (SD 52.35) from 72.06 (SD 56.47) and the left counterparts declined to 70.76 (SD 56.27) from 81.47 (SD 53.98). The WMFT quality improved significantly from mean of 1.45 (SD 1.14) to 2.13 (SD 1.39) and 1.24 (SD 1.01) to 1.61 (SD 1.28) in the right and left subgroups, respectively.
The ischemic and hemorrhagic subgroups also showed significant (p ≤ 0.001) changes on all the outcome measures for the postassessment in comparison to the prescores. The FMA-UE increased to mean of 39.57 (SD 16.17) from 28.66 (SD 13.37) and 28.88 (SD 17.68) from 22.05 (15.47 SD), respectively, in the ischemic and hemorrhagic subgroups. The WMFT-time declined to mean of 46.65 (SD 53.78) from 65.26 (SD 55.44) and 87.81 (SD 53.44) form 94.14 (SD 49.74), respectively, in the ischemic and hemorrhagic subgroups. Similarly, the WMFT-quality improved from mean of 1.52 (SD 0.99) to 2.11 (SD 1.27) and 1.05 (SD 1.03) to 1.45 (SD 1.28), respectively, in the ischemic and hemorrhagic subgroups. Additionally, the post-WMFT-time was significantly higher (p = 0.02) among the ischemic participants as compared with the hemorrhagic counterparts.
Discussion
In poststroke subjects, motor recovery occurs in a hierarchical sequential pattern.38 39 Considering the motor recovery process, a structured, stage-specific, and activity-based treatment protocol was developed in the SBMT investigation. A synergistic association between the movement patterns has been established in our previous research,20 which was considered as the foundation of SBMT regime. The protocol exhibited favorable change in motor recovery among the chronic stroke subjects.
Since the protocol was designed considering the BRS framework, all the activities of SBMT were challenging but achievable. All the activities were graded using the standard principles7 in a progressive pattern. In the present study, substantial number of subjects proceeded to higher stage of recovery after the intervention. This could be attributed to enhancement of normal synergy and inhibiting the abnormal linkage among the lower stage subjects whereas facilitation of out-of-synergy movement among the higher stage participants.40 41 42 The recovery of poststroke subjects usually plateaus after 1 year.21 43 In this investigation, the mean duration of stroke onset was 18 months and 75% subjects were below BRS-3. This may authenticate the favorable changes induced by SBMT in a single-group design. Thus, the SBMT program could be considered as a rehabilitation method having potential to induce motor recovery even among chronic stroke survivors. Although SBMT could enhance the timely performance of various WMFT tasks in all the subjects, the ischemic group exhibited more positive changes than the hemorrhagic subgroup. This could be attributed to better functional potential among ischemic stroke subjects in comparison to the hemorrhagic stroke.44 Postintervention, almost all the components of FMA improved significantly. The reflex response was almost similar for pre- and postintervention. Only one subject scored 2 for the reflex item prior to the intervention whereas all other participants scored 4 for the same. This could be due to the inclusion criteria of BRS-2 at which the reflex activities are usually present.38 The activities facilitated for the appearance of motor components at lower recovery stage. But position and constrain were provided to inhibit the stereotypical motor behavior; for instance, shoulder abduction was provided in supine position, maintaining elbow straight. This facilitated the motor control of elbow extension and shoulder abduction, considered to be most crucial for diminishing the deviated movement pattern of the upper extremity.45 46 47
The scapular movement was emphasized for each stage with various positions and procedures. The scapular elevation was practiced bilaterally in sitting position at BRS-A 3 whereas the same was trained in standing position with the shoulder externally rotated and extended at BRS-A 6. It has been observed that subjects with good scapular elevation had better recovery of arm and hand. The finding is also supported by other authors emphasizing the role of scapular control in enhancing reaching and grasping task.48 The wrist extension and mass grasp were the commonly observed items which showed changes after the SBMT. This could be attributed to the task demand for holding most of the objects utilized in the intervention. The FMA item of normal reflex could only be scored if the abnormal synergistic movement gets disappeared.26 49 Preintervention, majority of the subjects did not respond to the item. In addition to this, negligible number of subjects had complete scoring for the items of tremor, dysmetria, and time.
In literature, investigation using the concept of synergy in stroke is sparse. In a randomized trial on 30 stroke subjects, the Brunnstrom hand manipulation was found to be effective in augmenting hand recovery.20 Few studies used the concept through robotic systems for few individual motor components only. In a study conducted by Tropea et al8 the acute poststroke subjects had been provided reaching task using the robotic system. In an investigation performed by Dipietro et al,50 117 chronic poststroke subjects were trained using a robotic device. Postintervention, the subjects exhibited considerable independent control of shoulder and elbow movements. The change indicated better motor recovery attributing to modulation of abnormal synergy.
The muscle synergies of the shoulder and elbow improved after the intervention.8 The abnormal synergistic pattern such as flexor has been recommended to be targeted for enhancing the arm function.13 51 In SBMT, shoulder abduction was considered from lower to higher stage. The movement was trained in different positions as well as with varied resistance depending upon the motor recovery stage. The significance of shoulder abduction has been supported by various laboratory studies. The investigations have recommended the use of progressive abduction loading to reduce the impact of flexor synergy enhancing the reaching ability.40 45 52
Diverse treatment techniques such as motor relearning program (MRP), constraint-induced movement therapy (CIMT), robotic, and repetitive task training have been considered for stroke rehabilitation.53 MRP principles do not consider abnormal synergistic behavior, though the practice of missing components and completion of task is always the goal. CIMT carries very narrow inclusion criteria and has limited effects in stroke.54 Robotic therapy could improve arm recovery but it is very expensive and emphasize on few motor components only.50 55 Repetitive task training and bilateral training exhibited favorable results; however, inclusion of the lower stage subjects is a constraint for these techniques.56 SBMT comprises practice of missing synergistic motor components considered to be critical parts of motor tasks. The task performance may not enhance motor recovery without considering the missing motor components.57 The SBMT protocol is very economical with broad inclusion criteria. Further, the protocol considers all the motor components across every stage of stroke.
In the present study, a change of 9 on FMA-UE (more than established MCID in chronic stroke)30 was observed among the chronic subjects, which could be considered as a substantial and meaningful recovery. The repetitiveness and movement-specific protocol led to improvement of both time and quality of performance as exhibited by favorable changes on WMFT. The change on WFMT was also higher than the established MCID values.33
Subgroup analysis was not conducted due to less number of subjects at BRS stage 4 and above. Absolute constraint of the unwanted movements such as elbow flexion and trunk compensation was not possible in the protocol. Another limitation of the study was the inability to determine the retention of the treatment effect. Further refinement of protocol and testing on large sample is warranted. Sophisticated outcome measure such as motion analyzer and electromyography may also be utilized to analyze the minute and incremental changes.
Conclusion
The SBMT protocol may be considered as a feasible as well as effective intervention to facilitate motor recovery among poststroke hemiparetic subjects. The regime, based on framework of hierarchical motor recovery could be considered as a potential intervention for stroke rehabilitation. The utilization of synergistic linkage in the protocol should be further explored using kinematic and kinetic measures. The randomized controlled trials are further needed to endorse the outcome of the present investigation.
Conflict of Interest
None declared.
Funding This study was funded by the Department of Health Research, India.
References
- Differences in motor recovery between upper and lower limbs: does stroke subtype make the difference? Int J Rehabil Res. 2016;39(2):185-187.
- [Google Scholar]
- Cortex integrity relevance in muscle synergies in severe chronic stroke. Front Hum Neurosci. 2014;8:744.
- [Google Scholar]
- Brunnstrom's Movement Therapy in Hemiplegia: A Neurophysiological Approach. (2nd ed.). Philadelphia: JB Lippincott; 1992.
- [Google Scholar]
- Muscle synergies: implications for clinical evaluation and rehabilitation of movement. Top Spinal Cord Inj Rehabil. 2011;17(1):16-24.
- [Google Scholar]
- Interjoint coordination dynamics during reaching in stroke. Exp Brain Res. 2003;151(3):289-300.
- [Google Scholar]
- Occupational Therapy for Physical Dysfunction. (6 ed.). USA: Lippincott William & Wilkins Wolter Kluwer; 2008. eds.
- [Google Scholar]
- Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: a pilot study. J Neuroeng Rehabil. 2013;10:103.
- [Google Scholar]
- A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci. 2015;9:82.
- [Google Scholar]
- Neck rotation modulates flexion synergy torques, indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol. 2012;108(11):3096-3104.
- [Google Scholar]
- The effects of asymmetric tonic neck reflex during reaching movement following stroke: preliminary results. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:1581-1584.
- [Google Scholar]
- Robotic exoskeletons: a perspective for the rehabilitation of arm coordination in stroke patients. Front Hum Neurosci. 2014;8:947.
- [Google Scholar]
- Associated reactions in the hemiplegic arm. Scand J Rehabil Med. 1982;14(3):117-120.
- [Google Scholar]
- Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci U S A. 2009;106(46):19563-19568.
- [Google Scholar]
- Adult Hemiplegia: Evaluation and Treatment. (3rd ed.). Oxfordx:Butterworth-Heinemann; 1990.
- [Google Scholar]
- Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A. 2012;109(36):14652-14656.
- [Google Scholar]
- Effectiveness of the Bobath concept in the treatment of stroke: a systematic review. Disabil Rehabil. 2020;42(12):1636-1649.
- [Google Scholar]
- Prediction of functional outcomes in stroke patients: the role of motor patterns according to limb synergies. Aging Clin Exp Res. 2015;27(5):637-645.
- [Google Scholar]
- Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front Hum Neurosci. 2015;9:6.
- [Google Scholar]
- Relation between the upper extremity synergistic movement components and its implication for motor recovery in poststroke hemiparesis. Top Stroke Rehabil. 2012;19(6):545-555.
- [Google Scholar]
- Motor recovery of stroke patients after rehabilitation: one-year follow-up study. Int J Neurosci. 2017;127(1):37-43.
- [Google Scholar]
- Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Arch Phys Med Rehabil. 2016;97(4):575-581.
- [Google Scholar]
- Brunnstrom recovery stage and motricity index for the evaluation of upper extremity in stroke: analysis for correlation and responsiveness. Int J Rehabil Res. 2009;32(3):228-231.
- [Google Scholar]
- Ability for basic movement as an early predictor of functioning related to activities of daily living in stroke patients. Neurorehabil Neural Repair. 2007;21(4):353-357.
- [Google Scholar]
- Improving the utility of the Brunnstrom recovery stages in patients with stroke: validation and quantification. Medicine (Baltimore). 2016;95(31):e4508.
- [Google Scholar]
- The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13-31.
- [Google Scholar]
- Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63(10):1606-1610.
- [Google Scholar]
- The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232-240.
- [Google Scholar]
- Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73(7):447-454.
- [Google Scholar]
- Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791-798.
- [Google Scholar]
- The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82(6):750-755.
- [Google Scholar]
- Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32(7):1635-1639.
- [Google Scholar]
- Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil Neural Repair. 2009;23(5):429-434.
- [Google Scholar]
- Barthel index for stroke trials: development, properties, and application. Stroke. 2011;42(4):1146-1151.
- [Google Scholar]
- A comparison of the Barthel Index and the Functional Independence Measure as outcome measures in stroke rehabilitation: patterns of disability scale usage in clinical trials. Int J Rehabil Res. 2005;28(2):135-139.
- [Google Scholar]
- Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors: systematic review and meta-analysis. Stroke. 2013;44(2):462-468.
- [Google Scholar]
- Physical Rehabilitation Outcome Measures: A Guide to Enhanced Clinical Decision Making. (2nd ed.). Ontario: Canadian Physiotherapy Association; 2002.
- [Google Scholar]
- Hierarchical properties of the motor function sections of the Fugl-Meyer assessment scale for people after stroke: a retrospective study. Phys Ther. 2008;88(12):1554-1567.
- [Google Scholar]
- Are the hierarchical properties of the Fugl-Meyer assessment scale the same in acute stroke and chronic stroke? Phys Ther. 2014;94(7):977-986.
- [Google Scholar]
- Progressive abduction loading therapy with horizontal-plane viscous resistance targeting weakness and flexion synergy to treat upper limb function in chronic hemiparetic stroke: a randomized clinical trial. Front Neurol. 2018;9:71.
- [Google Scholar]
- Neuromuscular synergies in motor control in normal and poststroke individuals. Rev Neurosci. 2018;29(6):593-612.
- [Google Scholar]
- Reducing the impact of shoulder abduction loading on the classification of hand opening and grasping in individuals with poststroke flexion synergy. Front Bioeng Biotechnol. 2017;5:39.
- [Google Scholar]
- Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82(1):4-13.
- [Google Scholar]
- Differences in outcome and predictors between ischemic and intracerebral hemorrhage: the South London Stroke Register. Stroke. 2013;44(8):2174-2181.
- [Google Scholar]
- Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke. Clin Neurophysiol. 2017;128(7):1308-1314.
- [Google Scholar]
- Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 2010;41(4):745-750.
- [Google Scholar]
- Elbow extension predicts motor impairment and performance after stroke. Rehabil Res Pract. 2011;2011:381978.
- [Google Scholar]
- Characteristics of neuromuscular control of the scapula after stroke: a first exploration. Front Hum Neurosci. 2014;8:933.
- [Google Scholar]
- Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42(2):427-432.
- [Google Scholar]
- Muscle synergy space: learning model to create an optimal muscle synergy. Front Comput Neurosci. 2013;7:136.
- [Google Scholar]
- Robotic quantification of upper extremity loss of independent joint control or flexion synergy in individuals with hemiparetic stroke: a review of paradigms addressing the effects of shoulder abduction loading. J Neuroeng Rehabil. 2016;13(1):95.
- [Google Scholar]
- Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741-754.
- [Google Scholar]
- Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: systematic review and meta-analysis. Int J Rehabil Res. 2016;39(3):197-210.
- [Google Scholar]
- Review of the randomized clinical stroke rehabilitation trials in 2009. Med Sci Monit. 2011;17(2):RA25-RA43.
- [Google Scholar]
- Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med. 2009;52(3):269-293.
- [Google Scholar]
- Comparison of Brunnstrom movement therapy and Motor Relearning Program in rehabilitation of post-stroke hemiparetic hand: a randomized trial. J Bodyw Mov Ther. 2012;16(3):330-337.
- [Google Scholar]







