Translate this page into:
Surgical seeding of a chordoma into the temporal muscle
*Corresponding author: Basant K. Misra, Department of Neurosurgery and Gamma Knife Surgery, P D Hinduia National Hospital and Medical Research Centre, Mumbai, Maharashtra, India. basantkmisra@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hosmann A, Misra BK. Surgical seeding of a chordoma into the temporal muscle. J Neurosci Rural Pract. 2024;15:167-8. doi: 10.25259/JNRP_430_2023
Dear Editor,
Chordomas are rare malignant tumors of the axial skeleton representing <1% of intracranial tumors and 4% of all primary bone tumors.[1] Chordoma originates from the notochord and can be found along the cerebrospinal axis including the skull base (most frequently in the clivus), spine, and sacrococcygeal region.[2] These slow-growing tumors primarily develop extradurally within the bone but are invasive and locally destructive. Due to the proximity to critical neurovascular structures, achieving complete resection is often challenging. Chordomas are known for their resistance to both chemotherapy and radiotherapy and have a high tendency to recur locally even after treatment. Metastases to distant sites are found in 5–40% of cases and can affect various organs.[1] The most common sites of metastasis are the lungs, lymph nodes, liver, and bone. However, local seeding to adjacent muscles is an exceedingly rare occurrence.
A 73-year-old male patient presented 12-1/2 years ago with symptoms of bitemporal hemianopsia and vertigo. An MRI scan revealed a homogenously contrast-enhancing lesion in the sellar, suprasellar, and parasellar region [Figure 1a]. Subsequently, a right frontotemporal craniotomy was performed using a subtemporal zygomatic extradural approach to achieve a radical excision of the sellar/suprasellar tumor. The histopathology report confirmed the diagnosis of a chordoma. Five months later, residual tumor was treated with Gamma Knife radiosurgery (Leksell Gamma Knife® Elekta, AB, Sweden). The prescription dose was 16 Gy at 50% isodose, delivered through 12 shots.
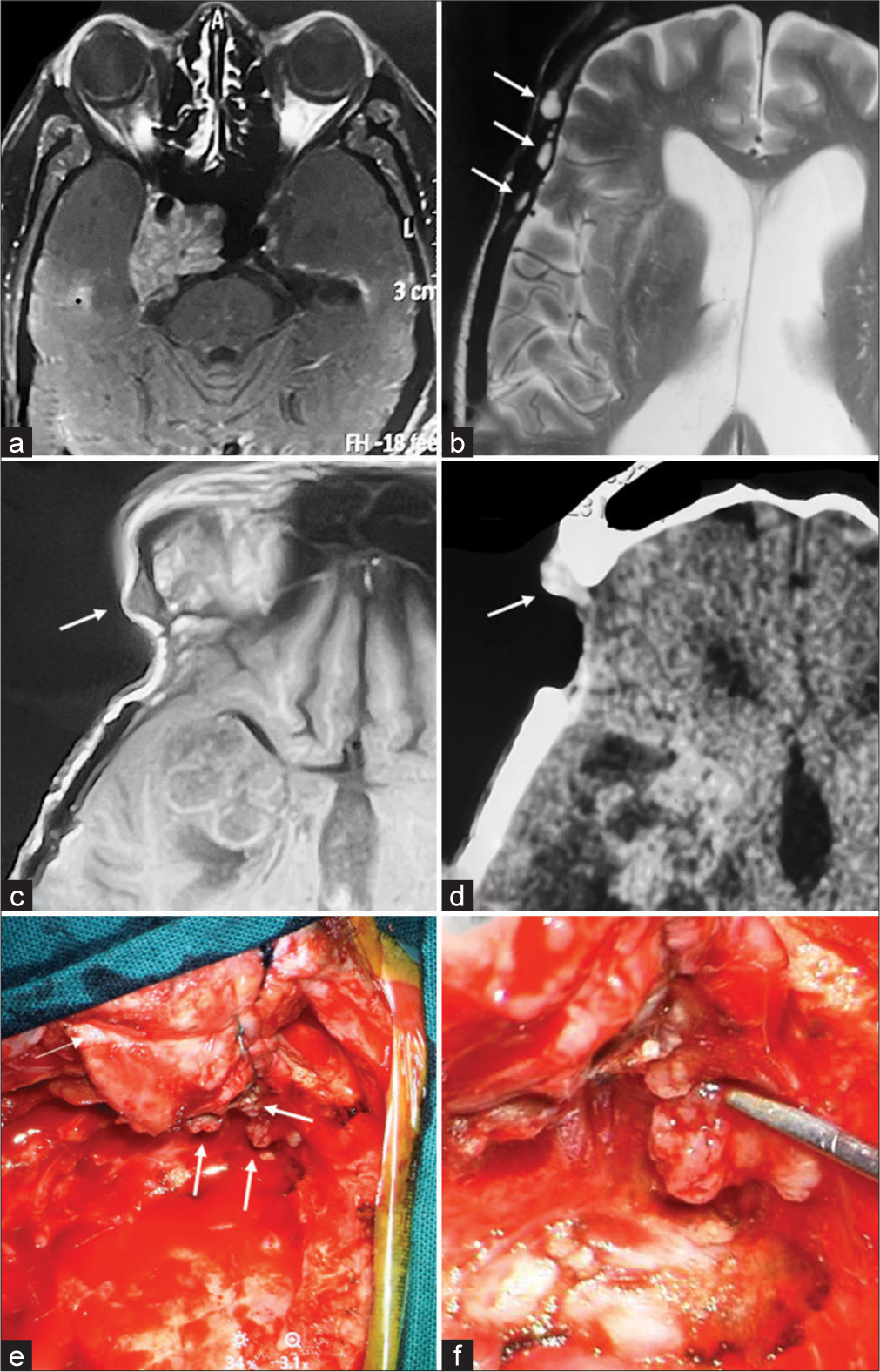
- (a) At the time of the initial diagnosis, magnetic resonance imaging (MRI) revealed a sizable contrast-enhancing lesion in the sellar, suprasellar, and parasellar region. After undergoing multiple resections for the chordoma, 12-1/2 years following the initial diagnosis and 20 months after the last craniotomy, T2- (b) and T1-weighted (c) MRI without contrast displayed multiple lesions within the temporal muscle. (d) Contrast enhancement was evident on CT scan. (e and f) During the dissection of the temporal muscle, multiple seedings of the chordoma were encountered. White arrows in part figures (b-e) indicate seedings of the chordoma.
During follow-up, the patient experienced recurrent tumor growth necessitating redo-surgeries at four, six, and nine years post-radiosurgery. Fifteen months after the last craniotomy, the patient underwent an extended transsphenoidal endoscopic radical decompression. Four months later, a ventriculoperitoneal shunt was implanted due to progressive hydrocephalus. Recently, 12-1/2 years since the initial diagnosis, the patient presented with complaints of generalized weakness and left-sided mild hemiparesis. Neuroradiological imaging at this time revealed local tumor recurrence as well as new contrast-enhancing lesions within the temporal muscle [Figure 1b-d].
Five months following the transsphenoidal resection and 20 months after the previous craniotomy, the patient underwent a subsequent right frontotemporal craniotomy to address the recurrence of the tumor. Intraoperatively, infiltrative seedings of the chordoma within the temporal muscle were observed, which were distinct and separate from the main tumor bulk. [Figure 1e and f]. These local metastases were surgically excised, and the tumor was radically resected.
While local tumor recurrence is often encountered, recurrent disease within the surgical pathway is a rare finding. Several case series have reported tumor recurrences along the surgical route in 1.2–7.3% of patients. Diagnosis of these recurrences typically occurs around 12 months after surgery with a range of 5–15 months.[3] Interestingly, in the present patient, local recurrence within the temporal muscle occurred quite late, 20 months after the last craniotomy. The most common locations for local recurrence, distant to the primary tumor, are the sinonasal cavity and palate after transsphenoidal resection.[3-5] Following transcranial resection, seedings are observed in the muscle and soft tissues directly beneath the surgical scar.[2,3] Moreover, surgical tumor seedings have been documented in the abdominal soft tissue where fat was harvested to serve as a graft during chordoma resection.[3] These findings suggest that the most probable mechanism for local tumor recurrence within the surgical pathway is the direct dissemination of tumor cells. Changing gloves and instruments after tumor resection, prior to wound closure, could potentially decrease the risk of spreading tumor cells. Furthermore, postoperative radiotherapy typically does not include the surgical pathway within the radiation field. This omission might elevate the risk of local tumor recurrence and warrants careful consideration in future treatment approaches.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Metastatic disease from spinal chordoma: A 10-year experience. J Neurosurg Spine. 2006;5:277-80.
- [CrossRef] [PubMed] [Google Scholar]
- The etiology of recurrent chordoma presenting as a neck mass: Metastasis vs. surgical pathway seeding. Ear Nose Throat J. 2008;87:106-9.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation therapy for chordomas of the base of skull and cervical spine: Patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579-84.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic seeding of skull base chordoma following endoscopic endonasal surgery. J Neurosurg. 2018;129:947-53.
- [CrossRef] [PubMed] [Google Scholar]





