Translate this page into:
Spontaneous rupture of arachnoid cyst
-
Received: ,
Accepted: ,
How to cite this article: Mamik HK, Kuldeep M, Sinha VD. Spontaneous rupture of arachnoid cyst. J Neurosci Rural Pract. doi: 10.25259/ JNRP_554_2023
Dear Editor,
Arachnoid cysts (ACs) are benign collections of cerebrospinal fluid (CSF), which represent a relatively small percentage of intracranial lesions.[1] Their incidence is reported at about 1% of all intracranial masses and is generally detected incidentally or on investigating symptoms after rupture.[1,2] With advancing imaging techniques such as magnetic resonance imaging (MRI) and increased availability of imaging, the reporting of ACs has increased in the past two decades. About 6% of these either present with rupture after trivial trauma or rupture after incidental diagnosis but spontaneous rupture of an AC is a very rare event.[3] On reviewing the available literature, we found that only six cases had been reported by the year 2000 and a total of 21 cases by the year 2004.[4] With greater utilization of imaging modalities, the reporting of incidental cases has increased, although spontaneously ruptured ACs are still limited to sporadic case reports due to the rarity of lesions and a few case series for suggestions on management.[1,5] Balestrino et al.[5] mentioned 57 reported cases with an additional series of 17 surgically managed cases in children in 2020. Massimi et al.[6] mentioned up to 106 cases of spontaneous rupture on retrospective analysis in 2022. Structurally, ACs are surrounded by an arachnoid sheet and may be primary (congenital) or secondary (to trauma, meningitis, or surgery).[7] They are more common in males and on the left side. Most ACs are located in the Sylvian fissure or middle cranial fossa (MCF) followed by posterior cranial fossa/retrocerebellar cistern and suprasellar cistern.[5] The usual complaint in most such cases of rupture is headache, which may be progressive or chronic.[1] In some cases, there may be contralateral hemiplegia.[8] A history of trauma, however trivial, must be actively sought.[9] Errors in diagnosis may result due to the rarity of the lesion and association with other neurological disorders like previous infarcts. The medicolegal aspects of such a scenario may result in medical malpractice or delay in treatment.[10] The Galassi classification[6] of MCF ACs was described in 1982 and divides these cysts into three types: (I) Small, spindle-shaped, and limited to the anterior portion of MCF, freely communicating with subarachnoid space (SAS); (II) superior extent along the Sylvian fissure, displacing temporal lobe, communicating with SAS; and (III) large cyst filling the MCF displacing frontal temporal and parietal lobes, often excluded from CSF communication with SAS.
Ruptured ACs may most commonly present as subdural hygromas, with or without neurological deficit or chronic or intracystic hematoma and signs of increased intracranial pressure (ICP).[1] This may result from a tearing of the membrane of the cyst, which may be spontaneous or attributable to reported or unreported trauma or manipulation. A focal increase in ICP during the Valsalva maneuver may also result in ruptured AC.[1] Risk factors for rupture include minor head trauma, diameter of more than 5 cm, and Galassi type 2 AC.[5] Due to the rarity of this lesion and the paucity of organized literature, the appropriate line of treatment remains uncertain. Conservative treatment includes watchful waiting and management with acetazolamide.[1,5] Surgical interventions including craniotomy or burr holes to drain subdural fluid, fenestration of the cyst into the basal cisterns for physiological clearance of CSF, and shunting of CSF by a cystoperitoneal shunt have also been attempted.[1,5] The presence of papilledema mandates emergent drainage of the subdural space. While shunting allows immediate decompression, it encumbers risks relating to shunt failure and infection. Microsurgical fenestration of cysts allows communication with CSF pathways and eliminates both acute compression and the risk of recurrent rupture.[5] Patients may be followed postoperatively and show a marked resolution of headaches and radiological changes.[1]
A 7-year-old male child, previously normal, presented with a history of progressive headache for one month with no seizures or antecedent trauma. The headache was spontaneous, to begin with, insidiously progressing to non-radiating holocranial moderate-to-severe pain. It had responded to intermittently given oral analgesics initially. The parents reported that the headache had become associated with multiple episodes of vomiting over the past few days. On examination, the child complained of headache but was alert and followed all commands without any focal motor deficit or cranial nerve involvement. His non-contrast computed tomography (CT) and MRI brain showed a left extra-axial perisylvian lesion along with an extension to the left cerebral convexity in a crescentic shape and a smaller right crescentic collection, hyperintense on T2 imaging, suggestive of a ruptured left temporal AC with bilateral subdural hygroma [Figure 1]. During surgery, drainage of the left subdural hygroma revealed a ruptured cystic lesion at the temporal tip. Left temporoparietal craniotomy with marsupialization of the cyst was done, and the cyst was connected to the basal cisterns. A safety burr hole was made on the right side in anticipation of the need for drainage of hygroma on the contralateral side. The cyst was sent for biopsy, which showed a cystic lesion composed of fibrocollagenous tissue lined with cuboidal to columnar epithelial cells [Figure 2]. Post-operative CT showed resolution of the cystic lesion with a decrease in subdural collection and no mass effect. The child recovered uneventfully and was routinely followed up in the outpatient department.
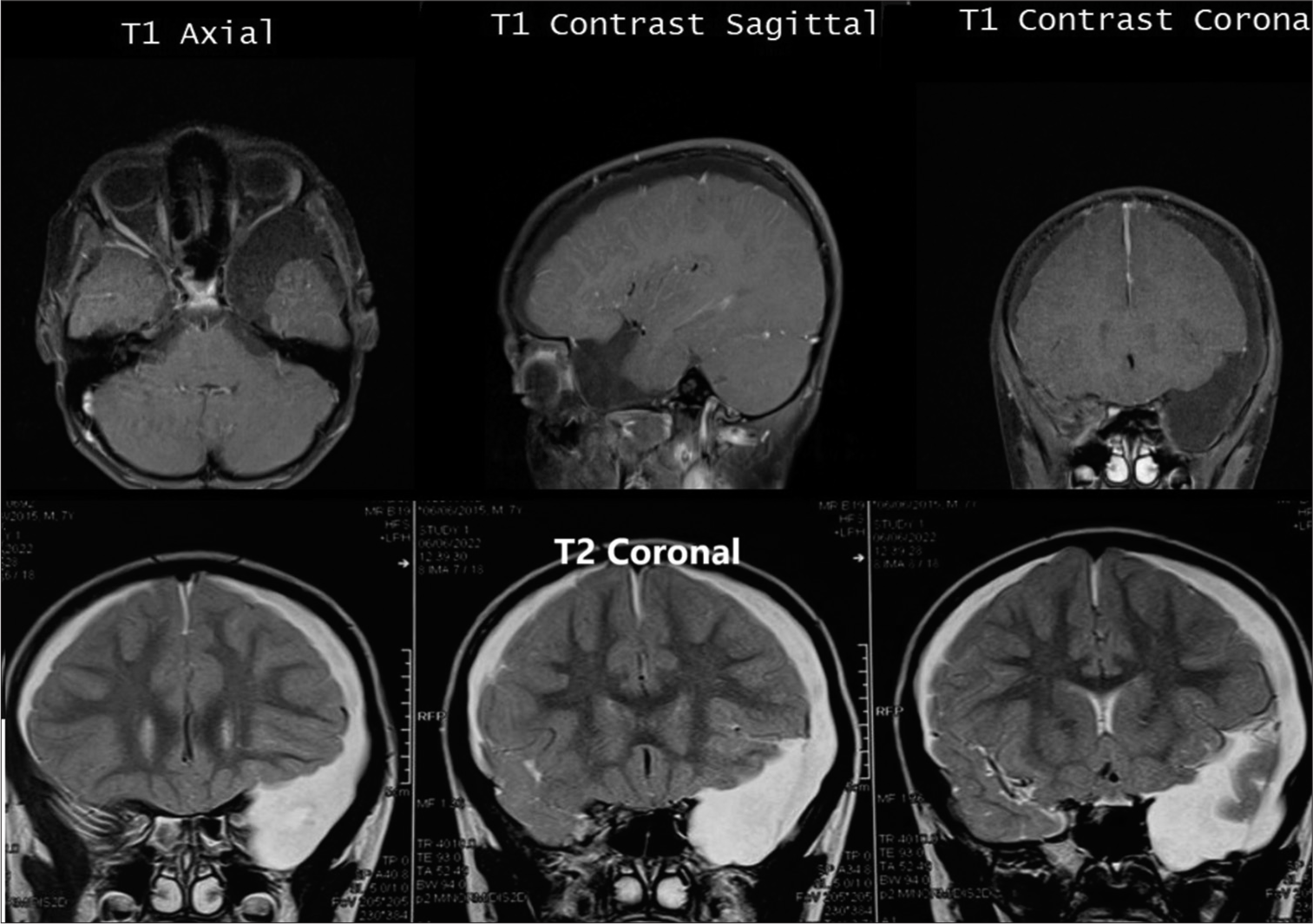
- Pre-operative magnetic resonance imaging (MRI) – T1-weighted image showing a ruptured arachnoid cyst in axial, sagittal, and coronal sections (upper) and pre-operative MRI – T2-weighted image showing a ruptured left temporal arachnoid cyst with bilateral subdural hygroma (lower).
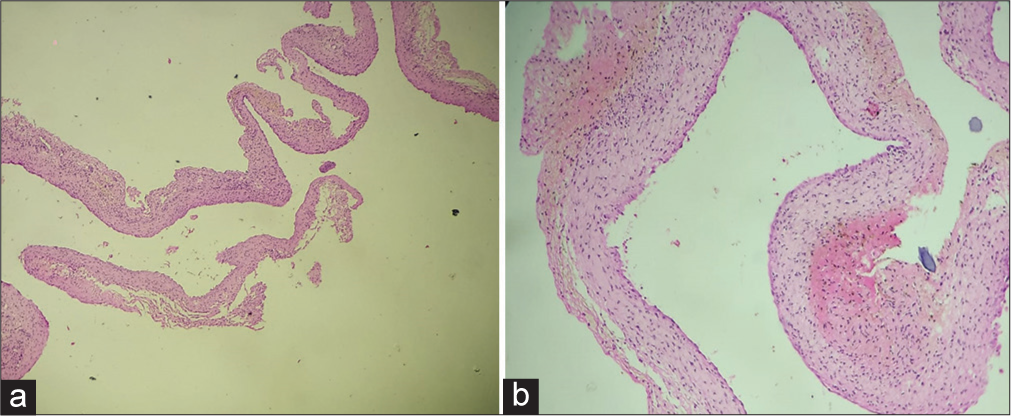
- Histopathological images showing fibrocollagenous tissue with cuboidal to columnar epithelial lining suggestive of arachnoid cyst in (a) low-power and (b) high-power fields.
Ruptured AC, though mostly an incidental imaging finding, is an important lesion with a high probability of misdiagnosis or delayed diagnosis.[10] It may rarely present with signs of raised ICP and warrant urgent drainage or shunting.[1] Available recommendations include weak evidence and case reports. Hence, further studies and targeted approaches are required for management.[5]
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Subdural hygroma after spontaneous rupture of an arachnoid cyst in a pediatric patient: A case report. Radiol Case Rep. 2020;16:309-11.
- [CrossRef] [PubMed] [Google Scholar]
- Arachnoid cyst rupture with subdural hygroma: Report of three cases and literature review. Childs Nerv Syst. 2002;18:609-13.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for pediatric arachnoid cyst rupture/hemorrhage: A case-control study. Neurosurgery. 2013;72:716-22.
- [CrossRef] [PubMed] [Google Scholar]
- Arachnoid cyst with spontaneous rupture into the subdural space. Br J Neurosurg. 2000;14:568-70.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous rupture of middle fossa arachnoid cysts: Surgical series from a single center pediatric hospital and literature review. Childs Nerv Syst. 2020;36:2789-99.
- [CrossRef] [PubMed] [Google Scholar]
- Ruptured Sylvian arachnoid cysts: An update on a real problem. Childs Nerv Syst. 2023;39:93-119.
- [CrossRef] [PubMed] [Google Scholar]
- An arachnoid cyst rupture complicated with subdural hygroma in a middle-aged woman: A case report and review of the literature. Egypt J Neurosurg. 2023;38:42.
- [CrossRef] [Google Scholar]
- A rare case of spontaneous arachnoid cyst rupture presenting as right hemiplegia and expressive aphasia in a pediatric patient. Children. 2021;8:78.
- [CrossRef] [PubMed] [Google Scholar]
- Arachnoid cyst rupture producing subdural hygroma and intracranial hypertension: Case reports. Neurosurgery. 1997;41:951-6.
- [CrossRef] [PubMed] [Google Scholar]
- Medicolegal aspects of arachnoid cysts In: Turgut M, Akhaddar A, Turgut AT, Hall WA, eds. Arachnoid cysts. Cham: Springer; 2023.
- [CrossRef] [Google Scholar]





