Translate this page into:
Is functional mobility associated with quality of sitting in cerebral palsy? A cross-sectional study
*Corresponding author: Shreekanth D. Karnad, Department of Physiotherapy, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India. shrikanth.dk@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Doctor K, Karnad S, Krishnan S, Narayan A, Nayak A. Is functional mobility associated with quality of sitting in cerebral palsy? A cross-sectional study. J Neurosci Rural Pract. 2024;15:286-92. doi: 10.25259/JNRP_516_2023
Abstract
Objectives:
A group of neuromuscular system anomalies associated with non-progressive issues in the developing fetal or newborn brain are known as cerebral palsy (CP). These abnormalities are typified by poor posture and motor development, which limits the execution of functional activities. Consequently, to achieve the same goals as peers who are typically developing, children with CP employ a variety of compensatory postures and techniques. Given that both sitting and mobility are essential for functioning, assessing each skill alone and in relation to the other is necessary. This study aims to determine if a child’s functional mobility affects their sitting ability.
Materials and Methods:
Twenty CP (Gross Motor Function Classification System [GMFCS] levels I and II) children, aged 6–12, were enrolled in the research. The level of sitting scale (LSS) and the modified timed up and go (mTUG) test were utilized to evaluate sitting and functional mobility, respectively.
Results:
The quality of sitting was shown to have a substantial effect on functional mobility, as a significant difference in mTUG durations was established between LSS levels (P < 0.001) and persisted when analyzed within the same GMFCS level (P = 0.007).
Conclusion:
The importance of trunk control in functional mobility can be inferred from the link between sitting quality and mobility. To improve children with CP’s functional mobility, this evidence may be utilized to design a well-informed and specific intervention program incorporating trunk control.
Keywords
Cerebral palsy
Sitting ability
Sitting quality
Functional mobility
Trunk control
INTRODUCTION
The prevalence of cerebral palsy (CP) is believed to be between 2 and 2.5 cases per 1000 live births worldwide and three cases per 1000 live births in India making it one of the most severe childhood illnesses.[1,2] It leads to chronic disability in children, affecting their physical, mental, social, and functional well-being.[2,3] “CP describes a group of disorders of the development of movement and posture causing activity limitation that is attributed to non-progressive disturbances that occurred in the developing fetal or infant brain.”[3] Due to these developmental disorders, children with CP have difficulty performing functional tasks such as sitting, transfers, and ambulation.[3]
Children with CP exhibit abnormal sitting alignment leading to compensatory postures during functional activity.[4,5] Furthermore, existing literature implies that they have poor neuromuscular control and lack of supraspinal tone modulation leading to strong single-muscle activations and increased muscle coactivation around a single joint.[6,7] These altered muscle activation patterns causing restricted movements and diminished selective motor control lead to poor movement patterns and postural adjustments in children with CP.[6,7] This further contributes to poor sitting ability, which causes impaired performances of static and dynamic activities in sitting.[6,8]
The aforementioned postural deficits in children with CP lead to impaired trunk movements during sitting, standing, and walking.[9] In addition, delayed onset of walking and altered gait patterns and parameters in terms of slower speed, shorter step lengths, and impaired balance, have been described in this population.[10-13] These factors contribute to decreased gait efficiency, evidenced by the head, arms, and trunk’s amplified mechanical effort, thus altering mobility.[14] Hence, these impaired locomotor and ambulatory functions lead to restricted ability to perform activities and limit social interaction and participation.[15-17]
Sitting and ambulation are major components for estimating functional ability.[18] Good sitting ability and postural control provide essential contributions to acquiring a stable base of support while performing dynamic activities. However, these components are reported to be poor in the CP population.[7] The International Classifications of Functioning, Disability, and Health proposes that a thorough understanding of health necessitates an examination of the interrelationships among several elements including physical structure and function, execution of everyday activities, and engagement in social involvement.[19] Hence, assessing the quality of sitting, functional mobility, and their association is paramount while planning rehabilitative programs for CP.
Although the literature suggests a possible influence of postural control impairments over deficits in function, few studies have assessed the same. Moreover, there is a paucity of information in the literature about the relationship between sitting quality and functional mobility in CP. Therefore, this study aims to determine whether functional mobility and sitting quality are associated with individuals with CP.
MATERIALS AND METHODS
Study design and subject profile
An analytical cross-sectional study was conducted in special schools and the Neuro-Sensory Developmental Unit of Mangaluru. The study is registered on the Clinical Trials Registry India (CTRI/2020/03/023902). Following approval by the Institutional Ethics Committee Kasturba Medical College, Mangaluru (IEC Ref No: IEC/KMC/MLR/11–19/586), this cross-sectional analytical study involved 20 children with CP recruited through purposive sampling. All children with CP who visited the Neuro-Sensory Developmental unit and special schools in Mangaluru between December 2019 and March 2021 were screened. Children diagnosed with CP aged 6–12 years were classified as gross motor function classification system (GMFCS) level II or above. Children with any visual and hearing impairments, structural deformities of the spine, and any lower-limb injuries in the past six months were excluded from the study. Children who matched the eligibility criteria and their parents/guardians consented to participation, followed by the child’s assent, were recruited for the study, as shown in Figure 1.
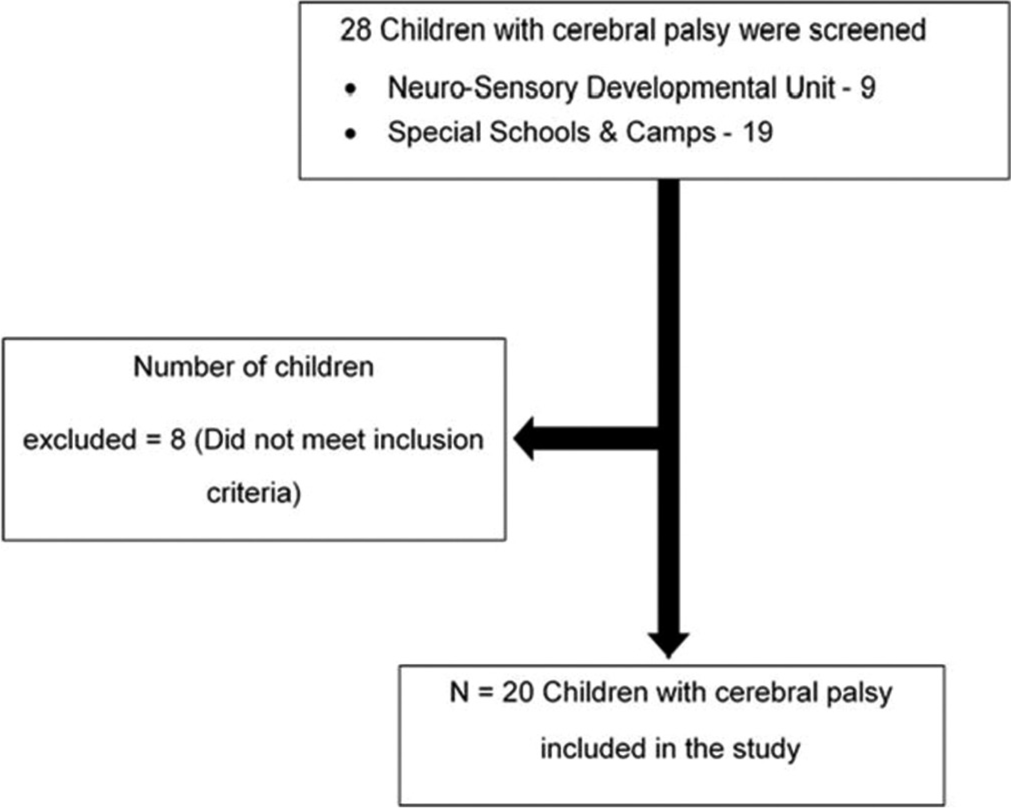
- Flow chart showing screening and recruitment of children for the study.
Sample size
With the variance s2 being 15.44 obtained from pilot study data analysis (Standard deviation [SD] = 3.93 of modified timed up and go [mTUG] test timings of 10 samples) and 95% confidence interval, the calculated sample size came to 16 using the following formula:
Z1-α/2 = 1.96 at 95% confidence interval
SD = 3.93; σ2 = (3.93)2 = 15.44
d = 2
Data collection tools and procedure
Level of sitting scale (LSS) for assessing the quality of sitting
Apparatus setup
A bench with the appropriate height such that the participants’ thighs were completely supported while the feet and trunk were unsupported was set up to permit trunk inclination.
Testing and data collection
The participant was asked/assisted to assume the standard sitting position defined in the LSS guidelines as follows:
The participants’ head position is either slightly flexed or neutral with respect to the trunk, and their hips and trunk are sufficiently flexed to allow the trunk to be inclined at least 60°. Considering the child’s comfort and safety, the position was held for at least 30 s. The individual was asked to shift his trunk and re-erect after maintaining the sitting position on his own for 30 s. The highest sitting level was recorded as Level 1 – Unplaceable: Child cannot be placed or held by one person in sitting position.; Level 2 – Supported from Head Downward; Level 3 – Supported from Shoulders or Trunk Downwards; Level 4 – Supported at Pelvis; Level 5 – Maintains Sitting Position independently; Level 6 – Shifts Trunk Forward (at least 20° without using hands), re-erects; Level 7 – Shifts Trunk Laterally (at least 20° without using hands), re-erects; and Level 8 – Shifts Trunk Backward (at least 20° without using hands), re-erects.
mTUG test for assessing functional mobility
Apparatus setup
A 3-m path free from obstruction was determined from a wall, and the distance was marked off with tape. A bench was placed at one end of the path, and a sticker was placed on the wall, which marked the other end of the walking distance. A stable bench befitting the participant’s height such that he/she was seated with feet completely supported on the floor was selected.
Testing and data collection
The mTUG test quantifies the duration, expressed in seconds, that a participant requires to transition from a seated position on a stool or bench, ambulate a distance of 3 meters, execute a turn, return to the original starting point, and resume a seated position. The participants were instructed to complete a predetermined activity, which involved touching a designated target, namely, a sticker, positioned on the wall at a distance of 3 m. During the assessment, the participants wore their regular footwear including modified footwear and orthosis. A preliminary trial was administered followed by the test being administered three times. The average of the three recorded durations was reported and utilized for analysis following the established rules of the mTUG.
Statistical analysis
The objective of the investigation was to ascertain the presence of any relationship between functional mobility and quality of sitting in children diagnosed with CP. The collected data was entered into the Statistical Package for the Social Sciences version 25.0. The data was tested for normality using the Shapiro-Wilk test and represented as Mean (SD). The distribution of 20 participants among the LSS levels (levels 7 and 8) was analyzed using Mann–Whitney’s U-test regarding demographic data. The association between functional mobility and sitting quality was analyzed by comparing the mean mTUG durations between LSS levels 7 and 8 using a t-test. A sub-group analysis was performed to understand if the association persists within each GMFCS level. Finally, Fisher’s Exact test was done to understand whether sitting or functional mobility quality varied with any demographic characteristics. A statistical significance was set at P < 0.05.
RESULTS
Demographic characteristics
The average age of the children in this study was 9.55 ± 2.24. Within the cohort of 20 youngsters included in this research investigation, it was observed that 12 individuals, constituting 60% of the sample, were male, while the remaining eight participants, accounting for 40% of the group, were female. In terms of ambulatory capacity, 5 (25%) of the participants were categorized as GMFCS I (ambulated independently and without limitations), and the remainder 15 (75%) ambulated with occasional assistance and minimal limitations (GMFCS II). Concerning LSS, our analysis showed that 11 (55%) of the sample was able to incline at least 20° in all four directions and re-erect (LSS 8), and the remainder 9 (45%) were able to incline at least 20° forward and laterally and re-erect (LSS 7). Furthermore, among the children who were classified as GMFCS II, 9 (60%) could incline their trunk forward and laterally and re-erect it (LSS 7) whereas 6 (40%) could incline their trunk in all four directions and re-erect to a neutral position (LSS 8). Statistical analysis revealed that mTUG varied only with GMFCS level (P = 0.003) while LSS varies with age (P = 0.001), gender (P = 0.04), and GMFCS level (P = 0.03).
Association between various variables
mTUG and LSS
The mean duration for completing the mTUG test for participants classified as LSS 7 and 8 was 15.36 ± 2.85 and 9.92 ± 2.18 s, respectively. The analysis observed a highly significant difference (P < 0.001) in the means of the mTUG duration when compared between LSS levels 7 and 8 as shown in Table 1 and represented in Figure 2.
| mTUG | |||||||
|---|---|---|---|---|---|---|---|
| LSS | n | Mean | Std. Deviation | 95% confidence interval for mean | t-test P value | Interpretation | |
| Lower bound | Upper bound | ||||||
| VII | 9 | 15.36 | 2.85 | 13.17 | 17.55 | 0.000* | HS@ |
| VIII | 11 | 9.92 | 2.18 | 8.46 | 11.39 | ||
| Total | 20 | 12.37 | 3.69 | 10.64 | 14.10 | ||
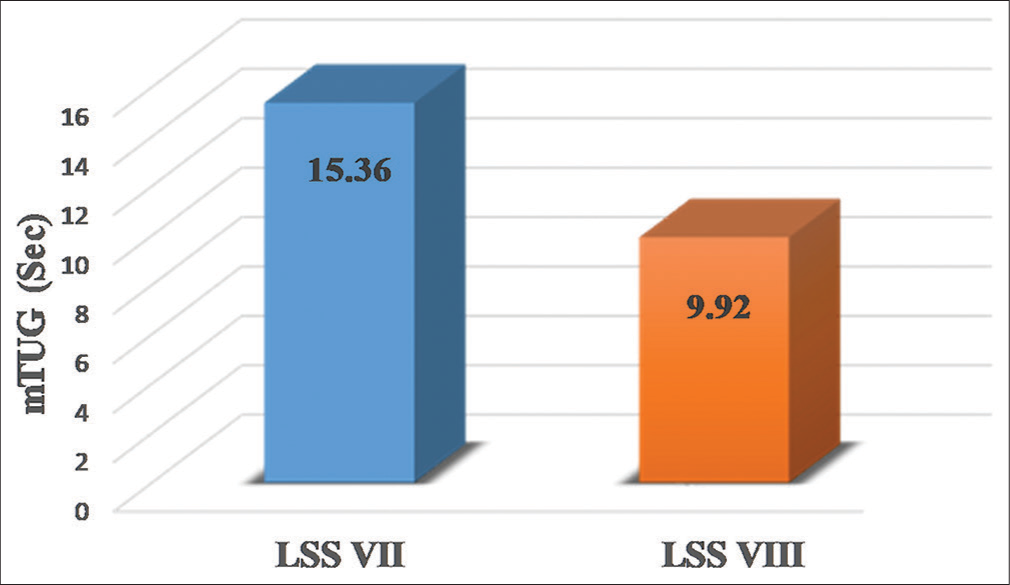
- Comparison of modified timed up and go (mTUG) test durations between level of sitting scale (LSS) levels.
A sub-group analysis further scrutinized the results to identify the difference in mTUG duration across LSS levels without the influence of GMFCS level (i.e., within each GMFCS level). The mean mTUG duration across both LSS levels was intended to be distinctly compared for each GMFCS level. However, since GMFCS level I only had participants classified as LSS 8, analysis for GMFCS I could not be performed. In contrast, the statistical analysis for GFMCS II revealed a highly significant difference between the mTUG duration across the LSS levels, and it remained so within the same GMFCS levels [Table 2].
| GMFCS level II | |||||
|---|---|---|---|---|---|
| LSS | n | Mean±SD | P-value | Interpretation | |
| mTUG | VII | 9 | 15.36±2.84 | 0.007* | HS@ |
| VIII | 6 | 11±2.12 | |||
mTUG and GMFCS
The mean mTUG duration for participants classified as GMFCS level I and II was 8.63 ± 1.55 and 13.62 ± 3.34 s, respectively. The statistical analysis showed a significant difference (P = 0.005), as represented in Figure 3.
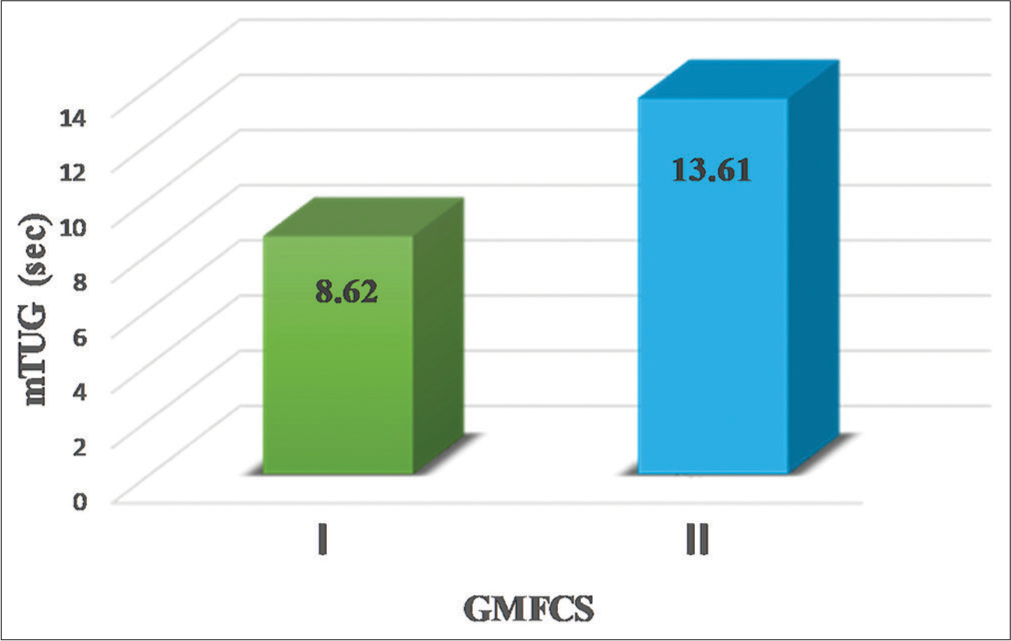
- Comparison of modified timed up and go (mTUG) test durations between gross motor function classification system (GMFCS) levels.
DISCUSSION
This study sought to understand whether the quality of sitting is associated with functional mobility in children with CP aged 6–12 years. The LSS has the potential to be used as a descriptive measure of the quality of sitting in neuromotor disorders and is very easily administered and feasible along with good test-retest and inter-rater reliability.[20,21] The mTUG Test has been identified as a reliable, valid, clinically feasible, quick, and easy-to-use measure of functional mobility in CP.[22,23] Various studies done in the past have strong evidence supporting the reliability and validity of mTUG in children with CP.[24-26]
For this study’s primary objective, the results indicate a statistically significant difference between the mTUG duration at LSS levels 7 and 8 suggesting a correlation between sitting quality and functional mobility. A similar result was described by Montero Mendoza et al. in 2015 where the authors established a relationship between sitting and walking ability.[27] However, Montero Mendoza et al. only considered ambulatory ability based on GMFCS classification.[27] In contrast, our study leaped a step further to understand functional mobility using the mTUG test, which includes functional elements such as sit-to-stand and stand-to-sit transitions, turning, and walking a specific distance. Additional information provided by this study was the presence of different sitting qualities among children with the same ambulatory ability (within the same GMFCS level). A review and meta-analysis in 2018 undertaken to establish prognostic predictors of ambulation in CP concluded that independent sitting achievement by two years is a significant predictor for ambulation in CP.[28]
The LSS fundamentally designates the descriptive quality of sitting for an individual based on the individual’s ability to maintain an erect sitting posture followed by an inclination of the trunk in various directions and returning to a neutral position, thus implying the role of trunk control in the quality of sitting.[21] Moreover, a noteworthy variation in the average time of the mTUG test among children belonging to the same GMFCS level suggests that trunk control plays a crucial role in functional mobility.
Children with CP show various extents of impaired static and dynamic trunk control.[7] The impaired trunk control leads to altered trunk movements and increased trunk sway during gait, manifested as altered bending and rotatory movements of the trunk.[11] In addition, children with CP show greater trunk accelerations during gait, which could be interpreted as a compensatory technique to lessen the increased center of mass (COM) excursion that arises during gait and is a consequence of diminished trunk control.[12,13] The increased excursion of COM leads to multiple compensatory mechanisms as there is an increased demand to maintain the COM within the base of support from an already poor postural control system as seen in children with CP.[11,29] Increased gait speed further accentuates these compensatory trunk accelerations.[13] To compensate for this, kids with CP walk more slowly than their peers, who are typically developing.[12] Increased excursions of COM and excessive trunk movements, secondary to impaired trunk control, make transitions such as sit-to-stand, turning around, and stand-to-sit difficult in children with CP.[30] These transitions are important components of functional mobility and are hence assessed as elements of the mTUG test. All the components mentioned above can be assumed to have played a role in functional mobility and influenced the mTUG duration of the participants. The mean mTUG duration of children with CP, as observed in this study, was 12.37 ± 3.69 s, higher than their typically developing peers (6.8 s ± 0.76 s) in the same age range.[31]
The study’s findings also show that sitting quality, as determined by LSS, varies with age and GMFCS level. One possible explanation for the relationship between LSS and age is that trunk control is segmentally acquired and grows progressively with age in the craniocaudal direction. This is demonstrated by improved sequential control at different trunk segments after the child starts sitting independently.[32] However, the many neuromuscular deficiencies associated with CP hinder the development of trunk control, which may be part of the reason why sitting quality is poor for children with CP.[29] The LSS varies with GMFCS levels and could be assumed to form the cornerstone of an association between sitting and mobility, as GMFCS levels are classified based on ambulatory ability. Furthermore, the literature suggests a threshold for trunk control between the GMFCS levels, and it is a possible principal factor distinguishing one GMFCS level from the other.[29] This further magnifies the role of trunk control in functional mobility, highlighting the association between sitting quality and functional mobility as observed in this study.
The study results suggest validation for the inclusion of strategies focusing on improving sitting quality to enhance functional mobility for children with CP. Including strategies focusing on trunk control could be most helpful, especially among children within the same GMFCS level where the therapist might overestimate their ambulatory ability without considering the pivotal role of the trunk on mobility. We propose that therapists in the future consider the trunk’s role among the principal focus areas for assessment and intervention while working on mobility. These findings would help improve gait capacity assessment and elaborate it by including trunk control and kinematics, especially among children with the same GMFCS level and thus presumed to have similar ambulation ability. Based on our results and the findings of previous studies, we suggest that planning intervention strategies to improve mobility should focus not only on lower extremity training but also on the quality of sitting and trunk control. This evidence can be used to plan an intervention with better precision, either focusing on rehabilitation to improve performance and functionality or providing compensatory adaptations for the dearth of control with the assistance of external devices. The study also sheds light on using LSS as a quick, feasible, and easily administered tool in a clinical setup for estimating functional mobility in children with CP. However, further research providing strong evidence for the same is warranted.
Limitations
While the present study delivers a unique input to understanding the association of functional mobility with the quality of sitting in children with CP, some crucial reflections are necessary. First, the trunk and lower extremities’ grading of spasticity and muscle strength were not considered, necessitating further investigation. Second, only children with higher functionality levels (GMFCS I and II) were included in the study; hence, children with lower functionality levels are not represented. Third, children with GMFCS level III ambulate with assistive devices, which could have confounded their usual gait parameters and mobility time when compared with higher GMFCS levels and were excluded from this study. Therefore, a separate study in the future focusing on children ambulating with the help of assistive devices could be undertaken. Fourth, the study used global test measures assessing posture and mobility as a whole. Future studies with specific test measures studying the specific aspects of posture (Static vs. Dynamic) and mobility (speed of walking, transitions, and stability of turns) should be done to provide more precise measures of relation and help enhance precision for the evaluation of interventional effectiveness. Fifth, the occurrence of association between the study parameters does not necessitate their causation trend. Consequently, future studies addressing interventional strategies for improving sitting quality by targeting trunk control and their impact on functional mobility could enhance our understanding.
CONCLUSION
Through this study, we intended to contribute new insight for assessment and rehabilitation strategies focused on enhancing functional mobility in children with CP. The study results could stipulate consideration for combined rehabilitation of trunk and lower extremities to enhance functional mobility in the CP population. It is hoped that this research will assist in making informed and precise clinical decisions while planning rehabilitation goals and strategies to enhance functional mobility in children with CP.
Authors’ contributions
The authors confirm their contribution to the paper as follows: Kaiorisa N. Doctor: Conceptualization, Methodology, Writing-Original draft preparation. Shreekanth D. Karnad: Conceptualization, Supervision, Data curation, Visualization, Investigation, Writing-Review and Editing. Shyam Krishnan: Supervision, Data curation, Visualisation. Amitesh Narayan: Supervision, Writing-Review and Editing. Akshatha Nayak: Writing- Reviewing and Editing.
Ethical approval
The research/study approved by the Institutional Review Board at Kasturba Medical College, Mangalore, number IEC/ KMC/MLR/11-19/586, dated 21/04/2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Study on the clinical profile of patients with cerebral palsy. IOSR J Dent Med Sci. 2016;15:54-8.
- [CrossRef] [Google Scholar]
- Etiopathological study on cerebral palsy and its management by Shashtika Shali Pinda Sweda and Samvardhana Ghrita. Ayu. 2013;34:56-62.
- [CrossRef] [PubMed] [Google Scholar]
- Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571-6.
- [CrossRef] [PubMed] [Google Scholar]
- Postural dysfunction in children with cerebral palsy: Some implications for therapeutic guidance. Neural Plast. 2005;12:221-8.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal sitting postures in children with neuromotor disabilities and use of the pelvic corset or molded seat for adaptative sitting. Pediatr Phys Ther. 1998;10:74-7.
- [CrossRef] [Google Scholar]
- Anticipatory and compensatory postural adjustments in sitting in children with cerebral palsy. Hum Mov Sci. 2011;30:648-57.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of impaired trunk control in children with spastic cerebral palsy. Res Dev Disabil. 2013;34:327-34.
- [CrossRef] [PubMed] [Google Scholar]
- A video based method to quantify posture of the head and trunk in sitting. Gait Posture. 2017;51:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between trunk control in sitting and during gait in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2015;57:344-50.
- [CrossRef] [PubMed] [Google Scholar]
- Natural progression of gait in children with cerebral palsy. J Pediatr Orthop. 2002;22:677-82.
- [CrossRef] [PubMed] [Google Scholar]
- Altered trunk movements during gait in children with spastic diplegia: Compensatory or underlying trunk control deficit? Res Dev Disabil. 2014;35:2044-52.
- [CrossRef] [PubMed] [Google Scholar]
- Stability and harmony of gait in children with cerebral palsy. Res Dev Disabil. 2012;33:129-35.
- [CrossRef] [PubMed] [Google Scholar]
- Gait characteristics in children and adolescents with cerebral palsy assessed with a trunk-worn accelerometer. Res Dev Disabil. 2014;35:1773-81.
- [CrossRef] [PubMed] [Google Scholar]
- Increased mechanical cost of walking in children with diplegia: The role of the passenger unit cannot be neglected. Res Dev Disabil. 2012;33:1996-2003.
- [CrossRef] [PubMed] [Google Scholar]
- Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248-57.
- [CrossRef] [PubMed] [Google Scholar]
- Postural asymmetries in young adults with cerebral palsy. Dev Med Child Neurol. 2013;55:1009-15.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disabil Rehabil. 2009;31:1971-9.
- [CrossRef] [PubMed] [Google Scholar]
- Functionality level and its relation to postural control during sitting-to-stand movement in children with cerebral palsy. Res Dev Disabil. 2014;35:506-11.
- [CrossRef] [PubMed] [Google Scholar]
- Parent-child interactions and children with cerebral palsy: An exploratory study investigating emotional availability, functional ability, and parent distress. Child Care Health Dev. 2017;43:812-22.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the relation between the type and amount of seating support provided and Level of Sitting Scale (LSS) scores for children with neuromotor disorders. Dev Neurorehabil. 2012;15:202-8.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a clinical measure of postural control for assessment of adaptive seating in children with neuromotor disabilities. Phys Ther. 1991;71:981-93.
- [CrossRef] [PubMed] [Google Scholar]
- Association timed “up and go” test with respect to gross motor function classification system level in children diagnosed as cerebral palsy. Int J Health Allied Sci. 2013;2:30-4.
- [CrossRef] [Google Scholar]
- Easy-to-use clinical measures of walking ability in children and adolescents with cerebral palsy: A systematic review. Disabil Rehabil. 2017;39:957-68.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability of “Modified timed up and go” test in children with cerebral palsy. J Pediatr Neurosci. 2012;7:96-100.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability and responsiveness of the timed up and go test in children with cerebral palsy. Pediatr Phys Ther. 2016;28:401-8.
- [CrossRef] [PubMed] [Google Scholar]
- The reliability and validity of the Timed Up and Go as a clinical tool in individuals with and without disabilities across a lifespan: A systematic review: Psychometric properties of the Timed Up and Go. Disabil Rehabil. 2019;25:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Association between gross motor function and postural control in sitting in children with Cerebral Palsy: A correlational study in Spain. BMC Pediatr. 2015;15:124.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic predictors for ambulation in children with cerebral palsy: A systematic review and meta-analysis of observational studies. Disabil Rehabil. 2018;40:135-43.
- [CrossRef] [PubMed] [Google Scholar]
- The central role of trunk control in the gross motor function of children with cerebral palsy: A retrospective cross-sectional study. Dev Med Child Neurol. 2015;57:351-7.
- [CrossRef] [PubMed] [Google Scholar]
- Postural control during sit-to-stand movement and its relationship with upright position in children with hemiplegic spastic cerebral palsy and in typically developing children. Braz J Phys Ther. 2015;19:18-25.
- [CrossRef] [PubMed] [Google Scholar]
- Timed up and go test (TUG): Reference data for Indian school age children. Indian J Pediatr. 2021;88:72.
- [CrossRef] [PubMed] [Google Scholar]
- The development of trunk control and its relation to reaching in infancy: A longitudinal study. Front Hum Neurosci. 2015;9:94.
- [CrossRef] [Google Scholar]







