Translate this page into:
Shape Profile of Corpus Callosum As a Signature to Phenotype Different Dementia
Sandhya Mangalore, MD, DM Department of Neuroimaging and Interventional Radiology, National Institute of Mental Health and Neurosciences Bangalore 560029 India drsandym@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Phenotyping dementia is always a complex task for a clinician. There is a need for more practical biomarkers to aid clinicians.
Objective The aim of the study is to investigate the shape profile of corpus callosum (CC) in different phenotypes of dementia.
Materials and Methods Our study included patients who underwent neuroimaging in our facility as a part of clinical evaluation for dementia referred from Geriatric Clinic (2017–2018). We have analyzed the shape of CC and interpreted the finding using a seven-segment division.
Results The sample included MPRAGE images of Alzheimer’ dementia (AD) (n = 24), posterior cortical atrophy- Alzheimer’ dementia (PCA-AD) (n = 7), behavioral variant of frontotemporal dementia (Bv-FTD) (n = 17), semantic variant frontotemporal dementia (Sv-FTD) (n = 11), progressive nonfluent aphasia (PNFA) (n = 4), Parkinson’s disease dementia (PDD) (n = 5), diffuse Lewy body dementia (n = 7), progressive supranuclear palsy (PSP) (n = 3), and corticobasal degeneration (CBD) (n = 3). We found in posterior dementias such as AD and PCA-AD that there was predominant atrophy of splenium of CC. In Bv-FTD, the genu and anterior half of the body of CC was atrophied, whereas in PNFA, PSP, PDD, and CBD there was atrophy of the body of CC giving a dumbbell like profile.
Conclusion Our study findings were in agreement with the anatomical cortical regions involved in different phenotypes of dementia. Our preliminary study highlighted potential usefulness of CC in the clinical setting for phenotyping dementia in addition to clinical history and robust biomarkers.
Keywords
dementia
phenotype
corpus callosum
white matter
subtype
Introduction
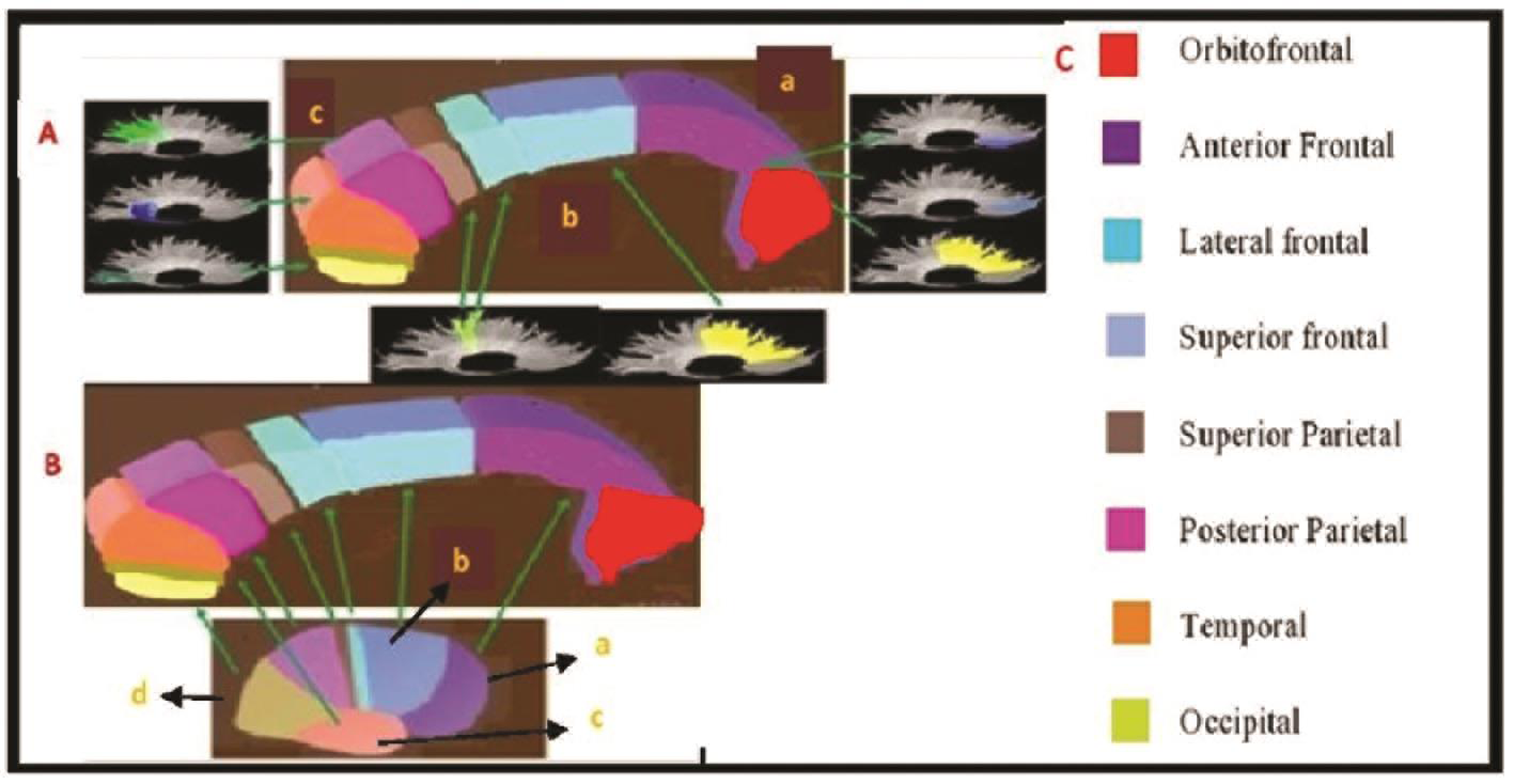
Cerebral hemispheres have interhemispheric connection served by corpus callosum (CC). CC reflects the topographic organization, connectivity of the fiber tracts and fiber tract sizes. Electrophysiology studies have elucidated CC and cortical lobe representation.1 Hence variation in tract connectivity and volumes is reflected in the CC. Anatomically prefrontal cortex fibers are represented in the genu and anterior part of the body. Premotor, motor, and sensory cortical connections are located in the three segments of the body of CC. Posterior parietal, temporal, occipital cortical connections are represented in the splenium of CC2 3 (Fig. 1). A recent diffuse tensor imaging-based study has observed an anteroposterior topography of interhemispheric tracts within the CC, consistent with neuroanatomical studies.4
-
Fig. 1 (A) Subdivisions of corpus callosum (CC) and white matter tracks—(a) rostrum and genu, (b) body, (c) splenium. (B) Gross lobar representation of CC (a) frontal, (b) parietal, (c) temporal, and (d) occipital lobe. (C) Corpus callosum fibers and their various cortical representation.
Fig. 1 (A) Subdivisions of corpus callosum (CC) and white matter tracks—(a) rostrum and genu, (b) body, (c) splenium. (B) Gross lobar representation of CC (a) frontal, (b) parietal, (c) temporal, and (d) occipital lobe. (C) Corpus callosum fibers and their various cortical representation.
The pyramidal neurons in layer III of cortical regions have axonal projections to the contralateral cerebral hemisphere that traverses through the CC. In neurodegenerative conditions, subsequent to neuronal cell death these axons undergo Wallerian degeneration and cause morphological/volumetric changes in CC.5 6 In support of above there were reports on CC segmental volume atrophy in mild cognitive impairment due to Alzheimer’ dementia (AD), frontotemporal dementia (FTD), Parkinson’s plus syndromes, and vascular dementia.7 8 9 10 11 In this way, CC anatomical maps with volume analysis has the potential to aid in the study of neurodegenerative and cognitive disorders as CC reflects the effects of neurodegeneration to specific cortical regions. Though the volume of each segment of CC gives more accurate information reflecting the cortical degenerative process, it has few limitations. These include being time-intensive, lack of normative data on CC segmental volume, variation in CC volumes in normal individuals, and more importantly unavailability of quantitative volumetric measurements in routine clinical practice.
In this scenario, the shape profile of CC can serve as an alternative to CC volume as a proxy marker for regional cortical degenerative process and thus help in phenotyping of dementia. CC shape profile of specific segments can be studied using more easily accessible imaging modality to clinicians such as magnetic resonance imaging/computed tomography-brain. However, there is paucity of studies on CC shape profile in various phenotypes of degenerative dementia.12 The objective of the current study is to investigate the profile of CC shape in different phenotypes of dementia.
Materials and Methods
Our study included patients who underwent advanced neuroimaging in our facility as part of clinical evaluation for dementia referred from geriatric clinic during 2017 to 2018. The imaging details were retrieved from the imaging hospital database using a unique identification number allotted to each patient. In all these cases the diagnosis was reached after a detailed evaluation and on the consensus of two psychiatrists specialized in geriatric psychiatry. The clinical diagnosis is based on DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) criteria and appropriate specific recent consensus criteria for each phenotype.13 14 15 16 17 Patients with atypical features of dementia and mixed etiology of dementia were excluded from the study. The current study is a part of a larger retrospective study on patients registered under geriatric clinic and services which has the approval of the Institutional Review Board. Anonymity of the patients was maintained using an alphanumeric code.
MRI-Brain Procedure and Image Acquisition
All examinations were performed on Philips 3T Ingenia scanner using a 32 channel head-neck-spine coil after obtaining an informed consent which is a standard operating procedure in our institute. A Standard magnetic resonance examination was performed including axial T1-weighted images, axial, coronal, and sagittal T2-weighted images as well as axial FLAIR (fluid attenuated inversion recovery) images, DWI, SWI, and followed by three-dimensional (3D)-T1 MPRAGE-weighted imaging. 3D T1 MPRAGE acquisition was with the following parameters: TR = 8.3 ms, TE = 3.8 ms, FOV = 24.6 cm, matrix = 248 × 246, slice thickness 1 mm without spacing, NEX =1.0. Midsagittal section of this image is used for visual analysis.
Sample
We have included MPRAGE brain-image for each phenotype of AD, posterior cortical atrophy (PCA), behavioral variant of frontotemporal dementia (Bv-FTD), semantic-FTD (SD), progressive nonfluent aphasia (PNFA), diffuse Lewy body dementia (DLBD), Parkinson’s disease dementia (PDD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD).
Corpus Callosum Segmental Division
We followed the seven-segment divisions of CC for our study.3 The genu forms the first part and occupies one-third of total CC volume followed by body, and splenium represents one-third of total volume, respectively. Body and splenium are further segmented into three parts (Fig. 1A). The interpretation of CC segments in relation to cortical representation is as per the Nordahl et al2 (Fig. 1B C).
Visual Interpretation
The mid-sagittal section of CC was used for analysis. The shape profile of CC was analyzed visually and interpreted the findings by two experts (S. M., S. V.: authors of study) in neuroimaging. S.M. and S.H. have independently reviewed the images and recorded the finding. The inter-rater reliability for the recorded finding was calculated using the kappa statistic. The kappa value was 0.741 indicating substantial agreement between the two raters.
Analysis
In each phenotype, the segment of CC involved was recorded and tabulated for further analysis. Depending on the most common CC segments involved (at least 50% of cases in each phenotype), we investigated the corresponding cortical regions involved. Subsequently, we compared the patterns of CC involvement in different phenotypes.
Results
Sample included 24 Alzheimer’s patients in our study. Among these there was atrophy of genu (25%), body (anterior: 25%, middle: 12.5%, posterior: 4.1%), and splenium (three divisions-79.2, 79.2, and 0%) (percent indicates proportion of patients). However, these are not exclusive with few patients noted to have overlapping features. On analysis, CC pattern atrophy with cortical regions in AD cases represented involvement of posterior parietal, temporal with few having prefrontal, premotor, and primary motor, primary somatosensory as part of generalized atrophy. In the seven PCA-AD, all cases had splenium atrophy in anterior third (100%) and 57.1% had atrophy of posterior part of body of CC. The segmental volume loss represented posterior parietal and few had primary somatosensory lobe as shown in Table 1.
|
Phenotype |
CC divisions involved in various conditions |
Corresponding cortical areas represented by CC |
Corresponding white matter tracts represented by CC |
|---|---|---|---|
|
Abbreviations: AD, Alzheimer’s dementia; Bv-FTD, behavioral variant of frontotemporal dementia; CBD, corticobasal degeneration; DLBD, diffuse Lewy body dementia; PCA, posterior cortical atrophy; PDD, Parkinson’s disease dementia; PNFA, progressive nonfluent aphasia; PSP, progressive supranuclear palsy; SD, semantic variant of FTD. |
|||
|
Normal |
Genu: one-third of total CC. 1. Genu Body: one-third of total CC. 2. Anterior third of body. 3. Mid third of body. 4. Posterior third of body. Splenium: 1/3 of total CC. 5. Anterior third of splenium. 6. Mid third of splenium. 7. Posterior third of splenium. |
1. Genu: one-third of total CC. Genu-prefrontal orbitofrontal, anterior frontal, small portion of lateral frontal, and medial (superior) frontal lobes. Body: one-third of total CC. 2. Anterior third of body-premotor (medial frontal lobes with small portion of lateral frontal lobe). 3. Mid third of body—primary motor. 4. Posterior third of body-primary somatosensory. Splenium: one-third of total CC. 5. Anterior third of splenium: posterior parietal. 6. Mid third of splenium: temporal. 7. Posterior third of splenium: occipital. |
Genu: one-third of total CC. 1. Genu: anterior frontal and orbitofrontal fibers and small representation by superior frontal fibers (medial frontal lobe). lateral frontal fibers (lateral frontal lobe). Body: one-third of total CC 2. Anterior third of body: superior frontal fibers. Small portion of lateral frontal fibers. 3. Mid third of body—superior parietal fibers. 4. Posterior third of body: superior parietal fibers. Splenium: one-third of total CC. 5. Anterior third of splenium: posterior parietal fibers. 6. Mid third of splenium: temporal fibers. 7. Posterior third of splenium: occipital fibers. |
|
AD (n = 24) |
1. Genu: 6/24 2. body: 6/24 3. body: 3/24 4. body: 1/24 5. splenium: 19/24 6. splenium: 19/24 7. splenium: 0. |
Posterior parietal, temporal few prefrontal, premotor, primary motor, primary somatosensory—generalized atrophy. |
Posterior parietal fibers, temporal fibers, and superior frontal fibers. |
|
PCA AD (n = 7) |
1. Genu: 0 2. body: 0 3. body: 0 4. body: 4/7 5. splenium: 7/7 6. splenium: 0 7. splenium: 0. |
All posterior parietal few primary somatosensory. More severe involvement of body than in AD. |
Superior parietal fibers, posterior parietal fibers. |
|
Bv-FTD (n = 17) |
1. Genu: 10/17 2. body: 10/17 3. body: 10/17 4. body: 10/17 5. splenium: 4/17 6. splenium: 4/17 7. splenium: 0. |
Prefrontal premotor, primary motor, primary somatosensory, lateral frontal lobe represented 1,2,3,4. |
Anterior frontal, orbitofrontal fibers, superior frontal fibers, lateral frontal fibers, superior parietal fibers. Few cases posterior parietal fibers and temporal fibers. |
|
SD (n = 11) |
8. Genu: 0 9. body: 9/11 10. body: 9/11 11. body: 7/11 12. splenium: 7/11 13. splenium: 2/11 14. splenium: 0. |
Premotor, primary motor, primary somatosensory, posterior parietal, temporal. |
Superior frontal fibers, superior parietal fibers, posterior parietal fibers, temporal fibers. |
|
PDD (n = 5) |
1. Genu: 1/5 2. body: 3/5 3. body: 3/5 4. body: 2/5 5. splenium: 2/5 6. splenium: 0 7. splenium: 0. |
Premotor, primary motor, and primary somatosensory and posterior parietal prefrontal. |
Superior frontal fibers, superior parietal fibers, posterior parietal fibers, anterior frontal, and orbitofrontal fibers. |
|
DLBD (n = 7) |
1. Genu: 2/7 2. body: 6/7 3. body: 0 4. body: 0 5. splenium: 4/7 6. splenium: 1/7 7. splenium: 0. |
Premotor, posterior parietal, prefrontal, temporal/occipital. |
Superior frontal fibers, posterior parietal few anterior frontal orbitofrontal fibers temporal fibers. |
|
PSP (n = 3) |
1. Genu: 3/3 2. body: 3/3 3. body: 2/3 4. body: 0 5. splenium: 1/3 6. splenium: 0 7. splenium: 0. |
Prefrontal, premotor, and primary motor, posterior parietal. Overall thinned body of CC except splenium. |
Superior frontal fibers, lateral frontal fibers, superior parietal fibers, posterior parietal fibers. |
|
CBD (n = 3) |
1. Genu: 1/3 2. body: 3/3 3. body: 0 4. body: 2/3 5. splenium: 3/3 6. splenium: 0 7. splenium: 0. |
Premotor, posterior parietal, primary somatosensory prefrontal. |
Superior frontal fibers, posterior parietal fibers, superior parietal fibers, anterior frontal fibers. |
We have included three FTD subtypes in our study. Among them, we analyzed 17 Bv-FTD, 11 SD, and four PNFA. In the Bv-FTD, there was atrophy of genu (58.8%), entire body (58.8%), and splenium (three divisions: 23.5, 23.5, and 0%). The CC segmental atrophy represented atrophy of prefrontal, premotor, primary motor, and primary somatosensory lobe. In the SD, there was atrophy of body of CC (anterior: 81.8%, middle: 81.8%, posterior: 63.6%) and splenium (three divisions: 63.6, 18.2, and 0%) with no atrophy of genu in any case. The involved segments represented premotor, primary motor, primary somatosensory, posterior parietal, and temporal lobe atrophy. In PNFA both the genu and splenium were spared with predominant involvement of the body of CC (anterior: 100%, middle: 75%, and posterior: 0%) (Table 1). The segments involved represented premotor, primary motor, and in few cases posterior parietal cortical regions.
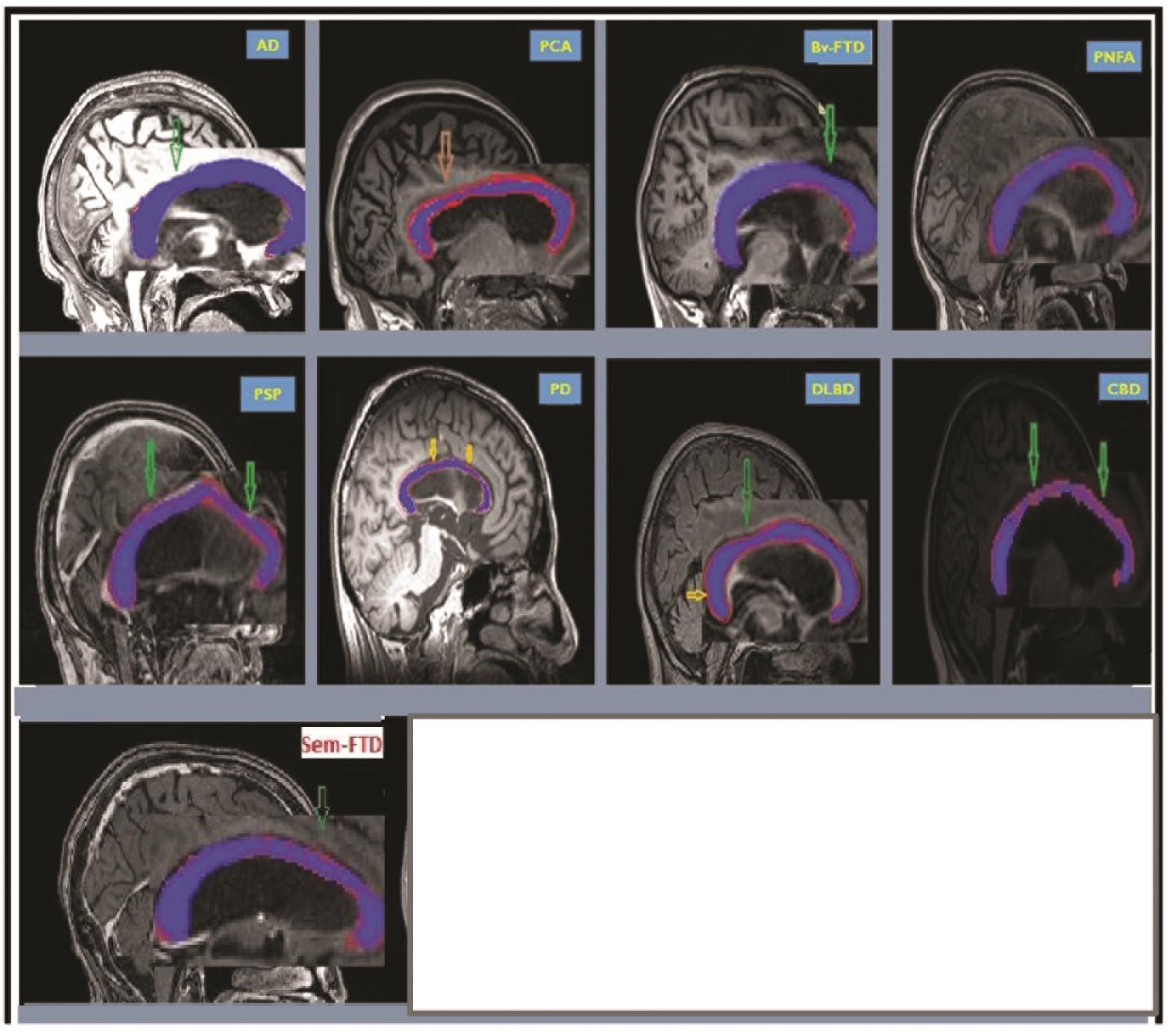
In other neurodegenerative conditions such as PD and other parkinsonian disorders, we evaluated five cases of PDD, seven cases of DLBD, three cases of PSP, and three cases of CBD. The pattern of atrophy of CC involved genu (20%) body (anterior: 60%, middle: 60%, posterior: 40%) and anterior third of splenium (40%). The segmental involvement in the PDD represented prefrontal, premotor, primary motor, and primary somatosensory and posterior parietal involvement. In DLBD there was atrophy of genu (28.6%), anterior half of body (85.7%), and splenium (three divisions: 57.1, 14.2, and 0%). The segmental pattern of CC atrophy in these cases represented premotor, posterior parietal, prefrontal, and temporal/occipital lobe involvement. In the PSP there was atrophy of genu (100%), body (anterior: 100%, middle: 66.7% and posterior: 0%), and anterior third of splenium (33.3%). CC segmental atrophy represented prefrontal, premotor and primary motor, and posterior parietal atrophy in PSP. In CBD, CC the pattern of atrophy involved genu (33.3%), body (anterior: 100%, middle: 0%, and posterior: 66.7%), and anterior third of splenium (100%) suggesting premotor, posterior parietal, primary somatosensory atrophy, and prefrontal atrophy (Table 1). The CC for each sample phenotype is highlighted as shown in Fig. 2.
-
Fig. 2 CC profile in AD, PCA, Bv-FTD, PNFA, SD, PSP, PD, DLBD, and CBD. CC is highlighted in each figure. AD, Alzheimer’s dementia; Bv-FTD, behavioral variant of frontotemporal dementia; CBD, corticobasal degeneration; CC, corpus callosum; DLBD, diffuse Lewy body dementia; PCA, posterior cortical atrophy; PD, Parkinson’s disease dementia; PNFA, progressive nonfluent aphasia; PSP, progressive supranuclear palsy; SD, semantic variant of frontotemporal dementia.
Fig. 2 CC profile in AD, PCA, Bv-FTD, PNFA, SD, PSP, PD, DLBD, and CBD. CC is highlighted in each figure. AD, Alzheimer’s dementia; Bv-FTD, behavioral variant of frontotemporal dementia; CBD, corticobasal degeneration; CC, corpus callosum; DLBD, diffuse Lewy body dementia; PCA, posterior cortical atrophy; PD, Parkinson’s disease dementia; PNFA, progressive nonfluent aphasia; PSP, progressive supranuclear palsy; SD, semantic variant of frontotemporal dementia.
Discussion
We felt the need for the study as we observed that in a clinical setting diagnosing phenotyping dementia is associated with certain challenges to a clinician.18 Phenotype of dementia is based on the historical temporal evolution of clinical symptoms, as it gives information on specific cortical regions involved and helps in phenotyping dementia. However, in many cases due to recall bias or lack of informants, it is often difficult to phenotype dementia clinically. Few others include cerebral comorbidity in degenerative dementia, systemic diseases, autoimmune conditions, and psychiatric conditions which can potentially confuse the clinical picture and phenotyping.19 20 21 22 23 In this situation, we felt CC shape profile can help as an easily available visual aid in the phenotyping dementia.
When we did an overall profile analysis, normal CC has an “eel-like” tubular appearance with a large bulbous Genu and a smaller bulbous splenium. The genu has a rostrum as a subunit which gives it a “beak-like” appearance. Though there are multiple overlaps of anatomical areas the gradient of severity of segmental involvement in the clinical context may act as a clue for underlying pathology.
Majority of earlier studies on AD investigated the volume and thickness of CC. These studies reported predominant involvement of posterior part of CC. Studies also found atrophy of posterior part of CC in MCI due to AD and progression in CC atrophy with the severity of AD.24 25 26 Our study was in agreement with previous studies with predominant involvement of the posterior part of CC (splenium). The involvement of specific CC segments in AD could be understood from the Wallerian degeneration of temporoparietal fibers which pass through the splenium.27 28 In comparison with AD versus PCA, there was more severe involvement of the body of CC in PCA compared with AD and helped to differentiate the phenotypes. Studies by Walterfang et al and Kaufer et al compared CC morphology among FTD and AD. These studies reported more consistent posterior involvement of CC in AD and anterior involvement of CC in FTD.12 29 Our findings were similar to the previous study on CC shape profile which helped in differentiating AD and FTD phenotypes. To the contrary, few studies did not find any difference in CC between AD and FTD.30
Fewer studies have looked at CC profile among the FTD subtypes. These studies have reported more diffuse involvement of CC in Bv-FTD, anterior part of body of CC in PNFA, whereas in SD there was minimal involvement of inferior genu compared controls.11 12 Our finding on CC profile in Bv-FTD is in agreement with above mentioned studies. The diffuse involvement of CC in Bv-FTD can be explained by the degenerative process of medial frontal and orbitofrontal cortex affecting anterior part of CC, degenerative process of superior and inferior temporal regions affecting the posterior part of CC.31 In PNFA, the involvement of left inferior frontal and insula reflected as atrophy of anterior and mid part of CC.32 Our finding in PNFA with sparing of genu and splenium and predominant involvement of body of CC was in agreement with previous studies. In SD our study reported involvement of body and anterior half of splenium in contrast to the inferior genu in previous studies.12 The minimal involvement of CC in PNFA could be explained by neuroanatomy of left anterior temporal poles with many of the axonal fibers traversing through the anterior commissure.33 However, overall, the subtle shape differences in CC could help in differentiating FTD subtypes along with other imaging markers.
In other neurodegenerative conditions such as PDD and other parkinsonian plus disorders, CC profiling may help to guide clinical diagnosis. The literature on CC in movements disorders is mostly on PD and PSP and very little on DLBD and CBS. Study by Goldman et al reported involvement on the mid-anterior and central part of CC in PD with similar picture noted in our PDD sample.8 Whereas, DLBD is a diffuse condition with bilateral involvement. The CC cannot differentiate unilateral involvement from bilateral involvement of cerebral hemisphere. Among the posterior dementia DLBD and AD are common. Apart from the clinical profile, CC also helps differentiation between the two. In AD there is atrophy of the entire body and entire splenial part, whereas in DLBD there is more pronounced atrophy of the first segment of body and splenium. The bulbosity of splenium is preserved in both cases. The sensorimotor fibers are relatively preserved in DLBD. However, there were few studies which did not find any difference in CC profile in PD, PDD, and DLBD compared with that in healthy controls.34 35
In our analysis in PSP, we found an overall thinning of body of CC giving an appearance of dumbbell as genu and splenia were spared. This is similar to the previous studies by Yamauchi et al which reported atrophy of the middle anterior part of CC.9 11 36 This helps in differentiating PSP from FTD and AD. Studies on CBD showed a similar pattern as in PSP with involvement of the middle part of CC.37 There is an overlap in terms of CC involvement in PD, PDD, DLBD, PSP, and CBD with subtle differences among them. Though CC helps in differentiating PD, PD plus syndromes from AD and FTD cannot differentiate PD and PD plus syndromes from each other. This is due to inherent limitations in CC as structural biomarker which will not identify unilateral or bilateral pathology and brainstem involvement. In these cases, clinical profile, imaging markers such as mid-brain will help in differentiating PD phenotypes.38
The basis for atrophy or narrowing of CC in CNS degenerative conditions is due to loss of gray matter neurons leading to secondary axonal loss.6 CC is also now hypothesized to be a pathway for abnormal proteins to spread across hemispheres such as amyotrophic lateral sclerosis.39 Few initial studies on neurodegenerative condition reported white matter microstructural changes prior to gray matter atrophy.40 However, it is difficult to study white matter atrophy in the clinical setting. Knowledge about the segmental anatomy of the CC and what it represents may help subtype these subtle tracts and cortical gray matter atrophy better.4
Certain confounders that can contribute to the variation in shape and thickness of CC include gender, handedness, and age-related white matter change.41 42 43 It is possible that some of the morphological changes of CC in our patients could have exaggerated or attenuated by above confounding factors which is a limitation. It was established that the degree of atrophy of CC depends on the duration and the severity of neurodegenerative condition.44 This was not taken into consideration which is a limitation of our study. Lack of control group in our study is another limitation.
To summarize in posterior dementias such as AD, AD-PCA corresponded to the atrophy of posterior part of CC. In Bv-FTD anterior part of CC was atrophied. In conditions such as PNFA, PDD, PSP, CBD, there is atrophy of body of CC. These findings are in agreement with the pathology of CNS regions. The atrophy pattern of SD is not in agreement with previous reports which need further study.
Implications
Findings of this paper open up the possibility for machine learning algorithms for each of the CC segment using its thickness, length shape profile as a biomarker. White matter has an important role in developing brain and in neurodegeneration. Though white matter atrophy represents a bidirectional pathology as part of Wallerian degeneration, it is an important structural biomarker.
Conclusion
Corpus callosum is a white matter bundle connecting both cerebral hemispheres. Any pathology to cortical gray matter results in changes in white matter and reflects in CC. In the current study, we explored the shape profile of CC in various phenotypes of dementia. Our visual analysis and interpretation of CC shown in different phenotypes of dementia have a unique shape profile of CC with few overlapping features. Our preliminary study highlights that CC shape profile along with comprehensive clinical history and examination can be useful in phenotyping dementia.
Future Direction
Taking our study findings forward, it is important to establish these results in future studies in a larger sample compared with age and gender matched healthy controls. It is also interesting for future studies to examine the CC changes in neurodegenerative conditions and its relationship with course of cognitive decline and neuropsychiatric manifestations.
Conflict of Interest
None declared.
Funding None.
References
- Localization of functional projections from corpus callosum to cerebral cortex. Chin Med J (Engl). 1991;104(10):851-857.
- [Google Scholar]
- Erratum: sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder (Molecular Autism (2015) 6 (26)) Mol Autism. 2015;6(1):39.
- [Google Scholar]
- Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799-835. (Pt 3)
- [Google Scholar]
- Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209(3):311-320.
- [Google Scholar]
- When, where, and how the corpus callosum changes in MCI and AD: a multimodal MRI study. Neurology. 2010;74(14):1136-1142.
- [Google Scholar]
- A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19(3):253-262.
- [Google Scholar]
- Corpus callosum atrophy rate in mild cognitive impairment and prodromal Alzheimer’s disease. J Alzheimers Dis. 2015;45(3):921-931.
- [Google Scholar]
- Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology. 2017;88(13):1265-1272.
- [Google Scholar]
- Role of corpus callosum volumetry in differentiating the subtypes of progressive supranuclear palsy and early Parkinson’s disease. Mov Disord Clin Pract (Hoboken). 2017;4(4):552-558.
- [Google Scholar]
- Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011;134:3264-3275. (Pt 11)
- [Google Scholar]
- Comparison of the pattern of atrophy of the corpus callosum in frontotemporal dementia, progressive supranuclear palsy, and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69(5):623-629.
- [Google Scholar]
- Shape analysis of the corpus callosum in Alzheimer’s disease and frontotemporal lobar degeneration subtypes. J Alzheimers Dis. 2014;40(4):897-906.
- [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. (5th ed). Philadelphia, PA: American Psychiatric Association; 2013.
- [Google Scholar]
- Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503.
- [Google Scholar]
- Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014.
- [Google Scholar]
- Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1-9.
- [Google Scholar]
- Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100.
- [Google Scholar]
- Phenotypic heterogeneity in dementia: a challenge for epidemiology and biomarker studies. Front Public Health. 2018;6:181.
- [Google Scholar]
- Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;12(1):192.
- [Google Scholar]
- The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287-1298. (Pt 5)
- [Google Scholar]
- Dementia and Alzheimer’s disease risks in patients with autoimmune disorders. Geriatr Gerontol Int. 2018;18(9):1350-1355.
- [Google Scholar]
- Multiple comorbid neuropathologies in the setting of Alzheimer’s disease neuropathology and implications for drug development. Alzheimers Dement (N Y). 2016;3(1):83-91.
- [Google Scholar]
- The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126-133.
- [Google Scholar]
- Callosal atrophy in mild cognitive impairment and Alzheimer’s disease: different effects in different stages. Neuroimage. 2010;49(1):141-149.
- [Google Scholar]
- Corpus callosum atrophy in patients with mild Alzheimer’s disease. Neurodegener Dis. 2011;8(6):476-482.
- [Google Scholar]
- Progression of corpus callosum atrophy in Alzheimer disease. Arch Neurol. 2002;59(2):243-248.
- [Google Scholar]
- Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630-653. (Pt 3)
- [Google Scholar]
- Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23(3):994-1005.
- [Google Scholar]
- Midline cerebral morphometry distinguishes frontotemporal dementia and Alzheimer’s disease. Neurology. 1997;48(4):978-985.
- [Google Scholar]
- Does the pattern of atrophy of the corpus callosum differ between patients with frontotemporal dementia and patients with Alzheimer’s disease? Dement Geriatr Cogn Disord. 2004;18(1):44-49.
- [Google Scholar]
- Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72(19):1653-1660.
- [Google Scholar]
- Cortical morphometric subclassification of frontotemporal lobar degeneration. AJNR Am J Neuroradiol. 2009;30(6):1233-1239.
- [Google Scholar]
- Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55(5):1174-1184.
- [Google Scholar]
- Diffusion tensor imaging in Alzheimer’s disease and dementia with Lewy bodies. Psychiatry Res. 2011;194(2):176-183.
- [Google Scholar]
- Corpus callosum in neurodegenerative diseases: findings in Parkinson’s disease. Dement Geriatr Cogn Disord. 2005;20(6):345-351.
- [Google Scholar]
- Atrophy of the corpus callosum, cognitive impairment, and cortical hypometabolism in progressive supranuclear palsy. Ann Neurol. 1997;41(5):606-614.
- [Google Scholar]
- Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol. 1998;55(5):609-614.
- [Google Scholar]
- Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21(2):714-724.
- [Google Scholar]
- Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127(3):423-439.
- [Google Scholar]
- White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822(3):416-422.
- [Google Scholar]
- Age and gender related changes in the dimensions of corpus callosum by MRI in South Indian population. Int J Anat Radiol Surg. 2017;6(3):RO47-RO51.
- [Google Scholar]
- The influence of sex, age, and handedness on corpus callosum morphology: a meta-analysis. Psychobiology (Austin Tex). 1995;23(3):240-247.
- [Google Scholar]
- The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neurosci Lett. 2003;351(2):99-102.
- [Google Scholar]
- Corpus callosum shape and size changes in early Alzheimer’s disease: a longitudinal MRI study using the OASIS brain database. J Alzheimers Dis. 2014;39(1):71-78.
- [Google Scholar]







