Translate this page into:
Role of Biofilm in Cerebrospinal Fluid Shunt Infections: A Study at Tertiary Neurocare Center from South India
Address for correspondence: Dr. R. Ravikumar, Department of Neuromicrobiology, National Institute of Mental Health and Neurosciences, Bengaluru - 560 029, Karnataka, India. E-mail: ravikumarbly@yahoo.co.uk
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Biofilms are the source of persistent infections of many pathogenic microbes. They are responsible for nosocomial infection and also associated with many surgical conditions including indwelling medical devices such as ventriculoperitoneal shunt. A significant problem encountered in shunt procedures is obstruction followed by infection, with infection rate ranging from 2% to 27%, often with poor outcome.
Materials and Methods:
This study was conducted in the Department of Neuromicrobiology at a tertiary neuroinstitute for 6 months from July 1 to December 31, 2014. The samples comprised cerebrospinal fluid (CSF) from suspected cases of shunt infections. Laboratory diagnosis of causative agent was established by adopting standard procedures. Then, isolates were evaluated for production of biofilm by tissue culture plate (TCP) method and tube method.
Results:
Of the 1642 shunt CSF samples obtained from neurosurgery, 14.79% were culture positive which yielded 254 isolates. About 51.97% were Gram-negative bacilli (GNB), 46.46% were Gram-positive cocci (GPC), and 1.57% were Candida albicans. Among GNB, nonfermenters were the most common (51.52%) followed by Pseudomonas aeruginosa (15.9%). Among GPC, coagulase-negative Staphylococci were 88.13%, out of which 43.26% were methicillin-resistant. Other GPC were Enterococcus spp. (4.24%), Staphylococcus aureus (5.08%), and Streptococcus spp. (2.54%). Among all isolates, 120 were tested for biofilm production, out of which 57.5% were biofilm producers and 42.5% were nonproducers.
Conclusions:
TCP was the better method to detect biofilm. Most of the biofilm producers were resistant pathogens.
Keywords
Biofilm
coagulase-negative Staphylococcus
shunt infection
tissue culture plate method
tube method
INTRODUCTION
Modern medical care has evolved in such a way that implantation of foreign bodies has become an indispensable part of it. Although they are used in all the fields of medicine for diagnostic and therapeutic procedure, they are important in managing critically ill patients.[1]
One of the most common procedures in neurosurgical practice is insertion of shunt, the mainstay of treatment for hydrocephalus over 50 years.[2] Common complications from ventriculoperitoneal (VP) shunt placement are intraventricular hemorrhage, obstruction, overdrainage of cerebrospinal fluid (CSF), and infection. Infection being the most serious, often requiring prompt management.[3] Infection rate ranges from 2% to 27%, coagulase-negative Staphylococcus (CONS) being the common organism associated.[4]
Among the complications of shunt procedures, shunt infections occur because of two main reasons; first, infection may be acquired during the surgery or the retrograde spread from peritoneum. Second, the shunt tube itself acts as nidus for infection by allowing organisms to form biofilm, by which they defend themselves from host immune response and antibiotics as well.[5] It is merely mandatory to remove the shunt device to eradicate infection since the organisms exist in biofilm status thereby raising their minimal inhibitory concentration 500 times more than the in vitro laboratory sensitivity result.[6]
Aims and objective
The aim of this study was to evaluate the infection rate, to isolate and identify the predominant pathogens, and to study the antibiotic sensitivity pattern of the organisms associated with VP shunt infections. To evaluate two methods for detection of biofilm formation in organisms isolated from shunt infections.
MATERIALS AND METHODS
The present study was conducted in the Department of Neuromicrobiology at a tertiary care neurocenter.
Source of data
CSF was collected from the suspected cases of shunt infection from neurosurgery ward and Neurosurgical Intensive Care Unit for 6 months from July to December 2014. The study comprised 1642 cases of clinically suspected cases of shunt infection.
After collection, CSF specimens were brought to the microbiology laboratory without delay and processed immediately.
Laboratory methods
After recording the macroscopic findings of CSF, cell counting was done followed by Gram staining. Bacterial and fungal cultures were put up, organisms were identified using standard biochemical tests, and antibiotic sensitivity was performed using Kirby-Bauer disk diffusion method using amikacin (30 μg), cefazolin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), cloxacillin (1 μg), colistin (10 μg), erythromycin (15 μg), gentamicin (10 μg), imipenem (10 μg), lincomycin (2 μg), linezolid (30 μg), ofloxacin (5 μg), oxacillin (1 μg), piperacillin (100 μg), piperacillin/tazobactam (110 μg), rifampin (5 μg), tetracycline (30 μg), tigecycline (15 μg), and trimethoprim/sulfamethoxazole (1.25/23.75 μg).[7] Interpretation was made according to the Clinical and Laboratory Standards Institute guidelines.[8]
Biofilm production
Biofilm production was evaluated by two methods, i.e., tube method (TM) and tissue culture plate (TCP) method.
Tube method
A qualitative assessment of biofilm formation was determined as previously described by Christensen et al.[9] Trypticase soy broth glucose (10 ml) was inoculated with loopful of microorganism from overnight culture plates and incubated for 24 h at 37°C. The tubes were decanted and washed with phosphate-buffered saline (PBS) (pH 7.3) and dried. Dried tubes were stained with crystal violet (0.1%). Excess stain was removed and tubes were washed with deionized water. Tubes were then dried in inverted position and observed for biofilm formation. Biofilm formation was considered positive when a visible film lined the wall and bottom of the tube. Ring formation at the liquid interface was not indicative of biofilm formation. Tubes were examined and the amount of biofilm formation was scored as 0 - absent, 1 - weak, 2 - moderate, or 3 - strong. Experiments were performed in triplicate and repeated 3 times.[10]
Tissue culture plate method
Isolates from fresh agar plates were inoculated in Trypticase soy broth with 1% glucose and incubated for 18 h at 37°C in stationary condition and diluted 1 in 100 with fresh medium. Individual wells of sterile, polystyrene, 96 well-flat bottom TCPs wells were filled with 0.2 ml aliquots of the diluted cultures, and only broth served as control to check sterility and nonspecific binding of media.
The TCPs were incubated for 24 h at 37°C. After incubation content of each well was gently removed by tapping the plates. The wells were washed 4 times with 0.2 ml of PBS (pH 7.2) to remove free-floating “planktonic” bacteria. Biofilms formed by adherent “sessile” organisms in plate were fixed with sodium acetate (2%) and stained with crystal violet (0.1% w/v). Excess stain was rinsed off by thorough washing with deionized water and plates were kept for drying. Adherent bacterial cells usually formed biofilm on all side wells and were uniformly stained with crystal violet. Optical density (OD) of stained adherent bacteria was determined with a micro ELISA auto reader (Tecan, Infinite M200) at wavelength of 570 nm (OD 570 nm). These OD values were considered as an index of bacteria adhering to surface and forming biofilms.
Experiment was performed in triplicate and repeated 3 times, the data were then averaged, and standard deviation was calculated. To compensate for background absorbance, OD readings from sterile medium, fixative, and dye were averaged and subtracted from all test values. The mean OD value obtained from media control well was deducted from all the test OD values.[10] Bacterial adherence classification by TCP method is shown in Table 1.

Statistical analysis was done by Chi-square test.
RESULTS
A total of CSF specimens received for culture and sensitivity from neurosurgery from suspected cases of shunt infections were 1642, of which 243 were culture positive indicating the infection rate as 14.79%. The cases ranged between 1 month and 75 years. A maximum number of cases were in the age group of 45–50 years, and a minimum number of cases were seen in 30–35 years. Out of 243 cases, 67.08% were male and 32.92% were female. The age distribution of cases is shown in Figure 1.
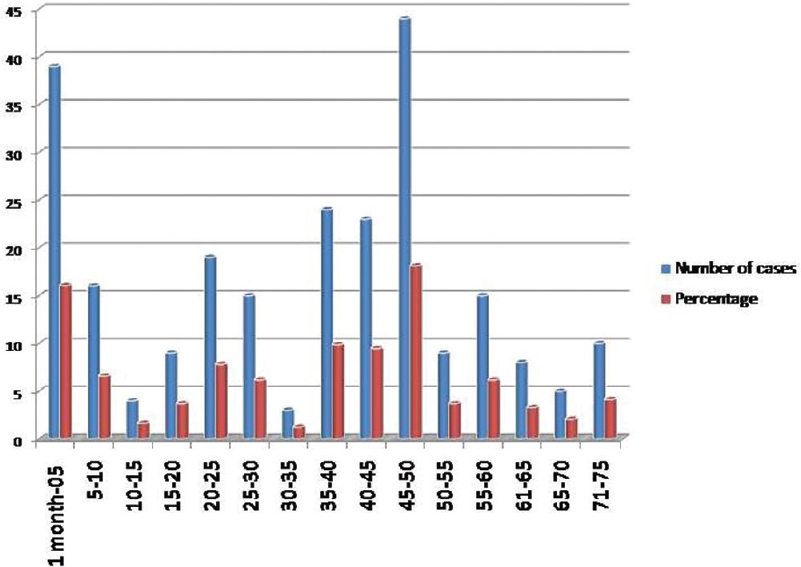
- Bar diagram showing age distribution of cases
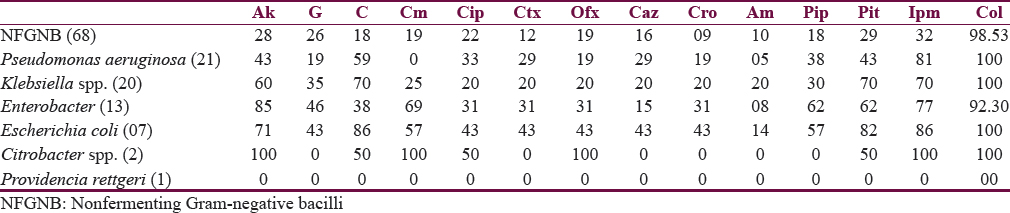
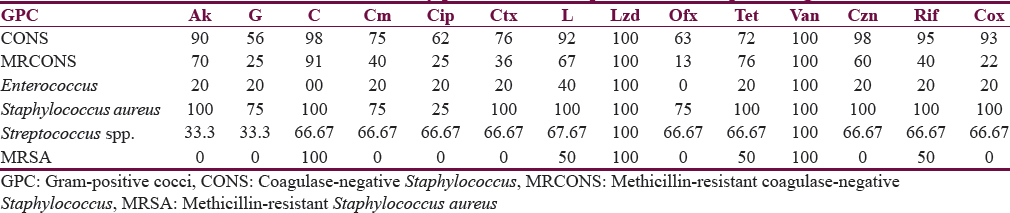
Two hundred forty-three samples yielded 254 isolates, of which 95.47% were monomicrobial and 4.53% were polymicrobial in nature. All the polymicrobial isolates were combination of two isolates. Out of 254 isolates, 51.97% were Gram-negative bacilli (GNB), 46.46% were Gram-positive cocci (GPC), and 1.57% were Candida albicans [Figure 2]. Among 132 GNB isolates, nonfermenting GNB (NFGNB) was the most common accounting for 51.52% [Figure 3]. Among 118 GPC isolates, CONS was the most common accounting for 50% [Figure 4]. The antibiotic sensitivity pattern of GNB is shown in Table 2 and GPC in Table 3.
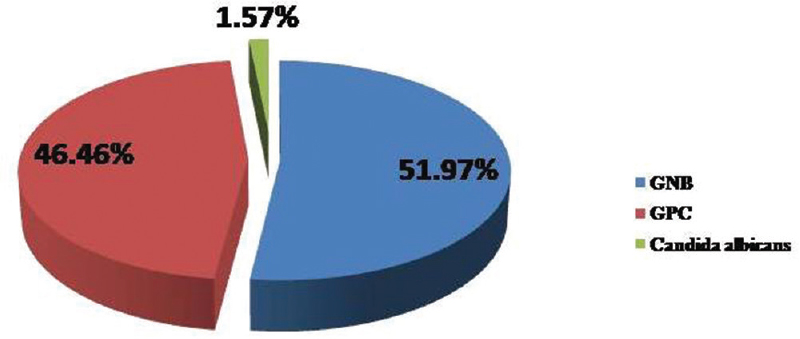
- Pie chart showing distribution of isolates
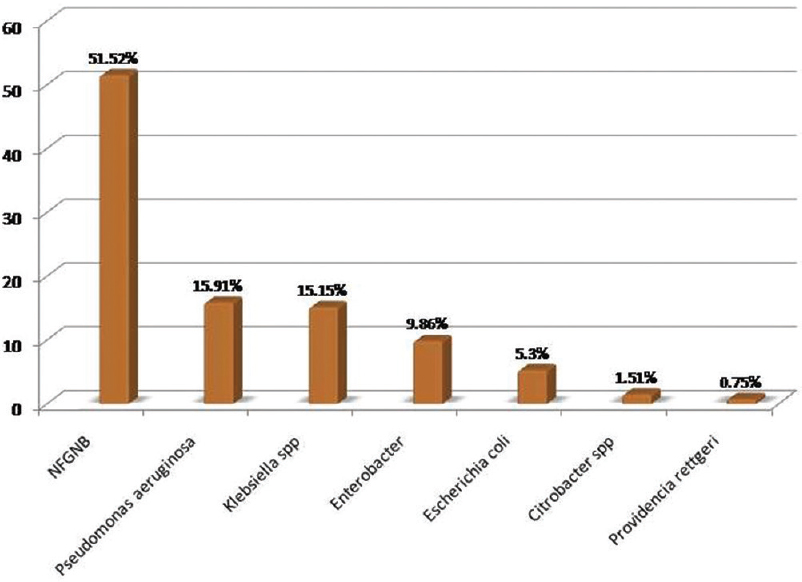
- Bar diagram showing distribution of Gram-negative bacilli
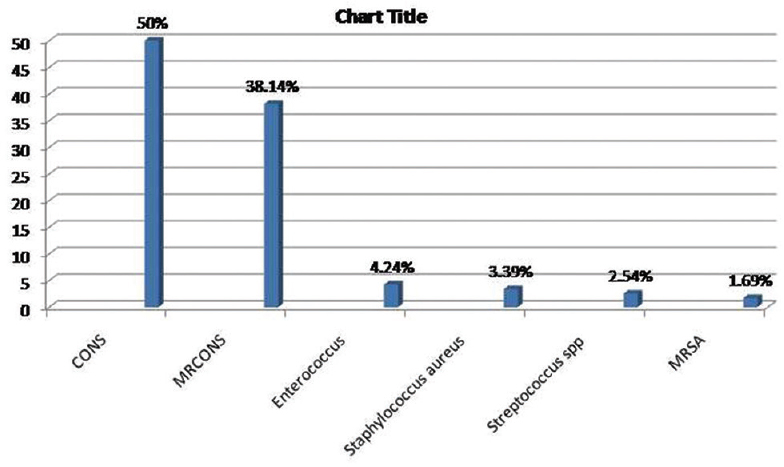
- Bar diagram showing distribution of Gram-positive cocci
Biofilm producers
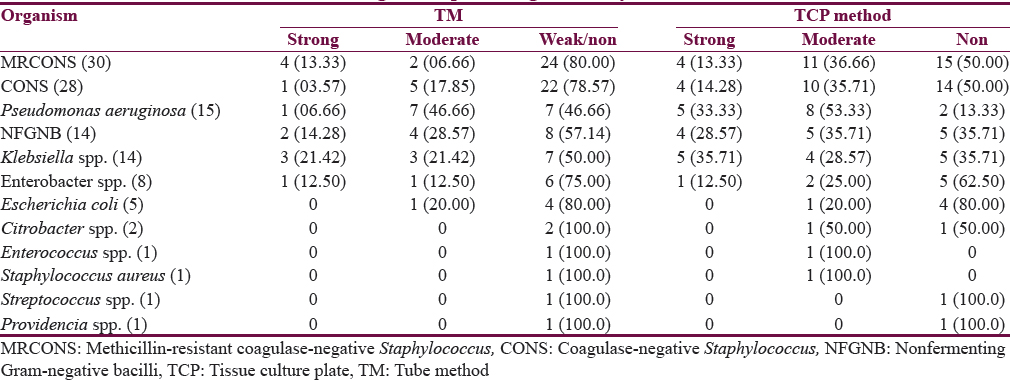
Out of 120 isolates, 55.83% were biofilm producers and 44.17% were nonproducers. In modified TCP method, among 120 isolates tested for biofilm formation, strong biofilm producers were 19.17%, 36.67% were moderate, and 44.16% isolates were considered as non or weak biofilm producers [Table 4]. In TM, out of 120 isolates tested for biofilm production, strong biofilm producers were 10%, of which 19.17% were moderate and 70.83% were non or weak biofilm producers [Table 4]. P value was significant (P < 0.05) indicating significant difference in results of two tests.

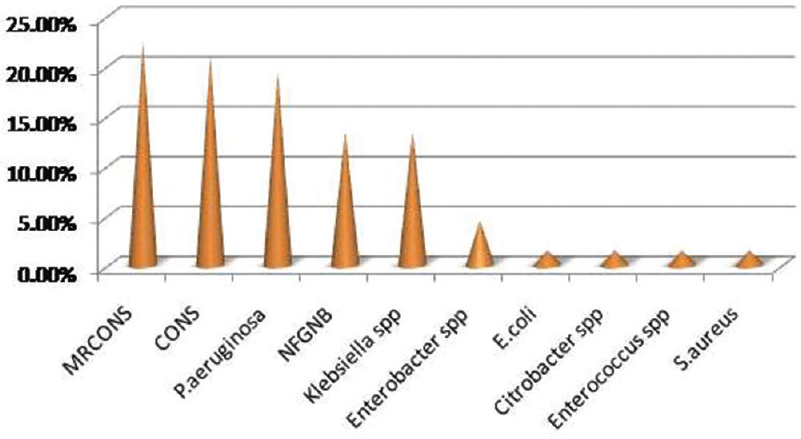
Out of 67 biofilm producers, the most common isolate to produce biofilm was methicillin-resistant coagulase-negative Staphylococcus (MRCONS) (22.39%) followed by CONS (20.90%) [Figure 5]. Detailed result of the biofilm producers by two methods is shown in Table 5.
- Bar diagram showing distribution of biofilm producing organisms
DISCUSSION
The first use of shunt device was credited to Nulsen and Spintz in 1952, they used rubber tube to divert the CSF from ventricle to jugular vein. Silicon tube was designed by John Holler for the treatment of his own hydrocephalic son. Then on words, shunt procedure has become one of the most common neurosurgical procedures; in some pediatric neurosurgical center, it accounts for nearly half of all the neurosurgical procedure carried out.[11]
Shunt procedure is associated with complications, the most common being obstruction followed by infections. Shunt infections are acquired during the procedure or immediately following the procedure.[11] The prevalence of shunt infections is in the range of 2%–27%.[4] The infection rate recorded by various authors like Bokhary and Kamal,[12] Sarguna and Lakshmi,[4] Choux et al.,[13] and Bierbrauer et al.[14] was 25.9%, 3.98%, 1.04%, and 5.21%, respectively. Such wide range of infection rates may be due to varying definitions of shunt infection and varying patient demographics reported throughout literature.[15]
Shunt infections were observed in wide range of age groups starting from newborn to elderly.[16] In the present study, shunt infections were most commonly seen in the age group of 45–50 years (44%) followed by 1 month to 5 years (39%). Out of 243 cases, 67.08% were male and 32.92% were female.
Most shunt infections are caused by Staphylococcus spp. with Staphylococcus epidermidis being the most common followed by Staphylococcus aureus and less commonly Enterococcus spp.[17] In our study, polymicrobial infection was seen in 4.53%. Out of 254 isolated pathogens, 51.97% were GNB, 46.46% were GPC, and 1.57% were C. albicans. The most common GPC isolated in our study was CONS (40.94%) followed by NFGNB (26.74%). CONS was the most common organism in studies by Sarguna and Lakshmi[4] and Ritz et al.[18] GNB and GPC represented 51.97% and 46.46% in our study compared to 7%–20% and 47%–80%, respectively, in other studies.[192021]
Shunt infection caused by GPC, majority of the organisms are commensals of the skin and can be a result of direct wound contamination during surgery. However, GNB colonization of skin is not so common; they could probably be introduced during surgery. Other GNB isolated in this study were enteric pathogens such as Escherichia coli, Enterobacter, and Citrobacter; this can be explained by an another possible mechanism, i.e., retrograde infection in which an asymmetric perforation of the bowel leads to contamination of distal VP shunt catheter and retrograde progression of infection.[222324]
Among GPC, CONS was quite sensitive to most of the antibiotics used while MRCONS has low sensitivity against gentamicin (25%), ciprofloxacin (25%), ofloxacin (13%), and cloxacillin (22%). S. aureus has got high sensitivity for most of the drugs except ciprofloxacin (25%); in contrast, 2 of the methicillin-resistant S. aureus isolated were sensitive only to chloramphenicol, linezolid, and vancomycin. Enterococcus spp. was resistant to all antibiotics except vancomycin and linezolid, whereas GNB isolated in this study have low sensitivity ranging from 19% to 50% to all the antibiotics used.
Biofilm is one of the important virulence factors for the organisms causing device-associated infections. Biofilm acts as nidus for organisms from where they may get detached and can cause blood stream infections, urinary tract infections; escape from action of host antibodies, produce endotoxin and generate resistant organisms.[25] They exhibit resistance to antibiotics by various methods such as restricted penetration of antibiotics into biofilms, decreased growth rate, and expression of resistant genes.[26] Bacteria commonly involved in biofilm production are CONS, S. aureus, E. coli, Klebsiella spp., and Pseudomonas spp.[27] Although there are many methods available for biofilm detection, standardized method has yet to be developed. These methods include TCP, TM, Congo red agar method, bioluminescent assay, light or fluorescent microscopic examination, air-liquid interface coverslip assay, and scanning electron microscopy.[28]
In our study, 120 isolates were tested for biofilm production, out of which 55.83% were biofilm producers and 44.17% were nonproducers. We followed and compared 2 methods, i.e., TM and TCP method. In TM of our study, 29.2% were biofilm producers as compared to TCP method in which 55.83% were biofilm producers.
Among the biofilm producers, MRCONS was the most common accounting for 22.39% followed by CONS (20.90%), Pseudomonas aeruginosa (19.41%) and NFGNB (13.43%). However, most of P. aeruginosa isolated were biofilm producers (86.66%), followed by Klebsiella spp. (64.28%) and NFGNB (64.28%). Among CONS and MRCONS, also 50% of them were biofilm producers indicating their most common pathogenic virulence factor as biofilm which needs further confirmation.
There are many studies comparing the different methods for detection of biofilm. In our study, TCP method detected more number of biofilm producers as compared to TM which is in concordance with studies by Mathur et al.,[10] Bose et al.,[29] Hassan et al.,[26] and Sharvari and Chitra;[27] however, in contrast, a study by Taj et al.[28] recommended TM. TM could detect a less number of strong biofilm producers as compared to TCP method. However, the interpretation is observer dependent, and there are chances of subjective errors. Further, it was difficult to differentiate between weak and nonbiofilm producers. This is in agreement with studies by Christensen et al.,[9] Mathur et al.,[10] and Sharvari and Chitra.[27]
Although there are highly specific and sensitive methods such as PCR analysis to detect ica gene as a virulence marker of biofilm, for developing countries like India, a low-cost method which requires less expensive equipment and technical expertise is needed. We suggest TCP method based on our findings. It is also reported as gold standard method by Mathur et al.[10]
The risk factor for the development of the shunt infection is intraoperative contaminations, others include holes in the surgical glove, postoperative CSF leak, younger age, and surgery by a neurosurgeon with limited experience.[3031]
Shunt infections can be prevented if periprocedural antibiotic prophylaxis is initiated and with the use of antibiotic-impregnated catheters (AIC). This technique is well documented in the literature by Pattavilakom et al.;[16] in their study, shunt infection was 6.5% before the institution of periprocedural antibiotic prophylaxis. They stated the concept of periprocedural antibiotic prophylaxis for antistaphylococcal antibiotic (vancomycin or flucloxacillin), the infection rate dropped to 6% with GPC amounting to 60% and GNB constituting 40%. In the next phase, they added ceftriaxone for GNB, but the infection rate increased to 7.7% majority are due to GPC. Once the AIC came to use, they used AIC impregnated with rifampicin and clindamycin along with ceftriaxone prophylaxis, only 3 cases out of 243 developed infections. This study highlights the simple methods for prevention of shunt infection. In an another study by Lane et al.[32] who compared antibiotic-impregnated shunts (AIS) and non-AIS (NAIS) in children. They concluded that there was no significant difference between two groups with respect to infection rate. The only difference was with respect to type of isolates; In AIS, the most common culturable pathogen was Gram negative bacilli and in NAIS it was Gram positive cocci. Hence, more studies are required to document the usefulness of AIS, especially in developing countries due to high cost.
The morbidity and mortality associated with shunt infection is very high. Hence, understanding the pathophysiology of causative microorganisms is important to prevent and manage shunt infections which requires a combined approach of neurosurgeon and clinical microbiologist.[1116]
CONCLUSIONS
In the present study, shunt infections constituted 14.79% with CONS being the most common organism. Among the tested isolates 55.83% were biofilm producer. TCP method is economical and gold standard for the detection of the biofilm production. Shunt infection is preventable with the use of periprocedural antibiotic prophylaxis and use of AIC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs. 2005;65:179-214.
- [Google Scholar]
- CSF shunts 50 years on – Past, present and future. Childs Nerv Syst. 2000;16:800-4.
- [Google Scholar]
- Hydrocephalus Indian scenario – A review. J Pediatr Neurosci. 2011;6(Suppl 1):S11-22.
- [Google Scholar]
- A protocol for diagnosis and management of cerebrospinal shunt infections and other infectious conditions in neurosurgical practice. Basic Clin Neurosci. 2012;3:61-9.
- [Google Scholar]
- Laboratory control of antimicrobial therapy. In: Fraser AG, Collee JG, Marmion BP, Simmons A, eds. Mackie and McCartney Practical Medical Microbiology (14th ed). New York: Churchill Livingstone; 1996. p. :151-78.
- [Google Scholar]
- Clinical Laboratory Standards Institute. Criteria for Laboratory Testing and Diagnosis of Human Immunodeficiency Virus Infection: Approved Guideline. CLSI Document M53-A. Wayne, PA: Clinical Laboratory Standards Institute; 2011.
- Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996-1006.
- [Google Scholar]
- Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24:25-9.
- [Google Scholar]
- Evaluation and management of shunt infections in children with hydrocephalus. Clin Pediatr (Phila). 2006;45:705-13.
- [Google Scholar]
- Ventriculo-peritoneal shunt infections in infants and children. Libyan J Med. 2008;3:20-2.
- [Google Scholar]
- Shunt implantation: Reducing the incidence of shunt infection. J Neurosurg. 1992;77:875-80.
- [Google Scholar]
- A prospective, randomized study of shunt function and infections as a function of shunt placement. Pediatr Neurosurg 1990. 1991;16:287-91.
- [Google Scholar]
- Defining bacterial meningitis and other infections of the central nervous system. Pediatr Crit Care Med. 2005;6(3 Suppl):S14-8.
- [Google Scholar]
- Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: An Australian prospective study. J Clin Neurosci. 2007;14:526-31.
- [Google Scholar]
- Action of linezolid or vancomycin on biofilms in ventriculoperitoneal shunts in vitro. Antimicrob Agents Chemother. 2012;56:2842-5.
- [Google Scholar]
- Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;7:38.
- [Google Scholar]
- CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138:1103-8.
- [Google Scholar]
- Cerebrospinal fluid shunt infections. A review of 35 infections in 32 patients. J Neurosurg. 1983;59:389-94.
- [Google Scholar]
- A study of sources of infection in colonized cerebrospinal fluid shunt infections. Influences on initial shunts. Dev Med Child Neurol. 1974;16:16-22.
- [Google Scholar]
- Ventriculoperitoneal shunt infections with gram-negative bacteria. Neurosurgery. 1993;33:858-62.
- [Google Scholar]
- Asymptomatic perforated viscus and gram-negative ventriculitis as a complication of valve-regulated ventriculoperitoneal shunts. Report of two cases. J Neurosurg. 1972;37:616-8.
- [Google Scholar]
- Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis. 2011;15:305-11.
- [Google Scholar]
- Evaluation of different detection methods of biofilm formation in clinical isolates of staphylococci. Int J Pharm Bio Sci. 2012;3:724-33.
- [Google Scholar]
- Study on biofilm-forming properties of clinical isolates of Staphylococcus aureus. J Infect Dev Ctries. 2012;6:403-9.
- [Google Scholar]
- Detection of biofilm producing Staphylococci: Need of the hour. J Clin Diagn Res. 2009;3:1915-20.
- [Google Scholar]
- Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51:804-11.
- [Google Scholar]
- Cerebrospinal fluid shunt infection: A prospective study of risk factors. J Neurosurg. 2001;94:195-201.
- [Google Scholar]
- Effectiveness of the bactiseal universal shunt for reducing shunt infection in a Sub-Saharan African context: A retrospective cohort study in 160 Ugandan children. J Neurosurg Pediatr. 2014;13:140-4.
- [Google Scholar]






