Translate this page into:
Rescue Measure in Giant Intracranial Meningioma Resection by Tranexamic Acid
Address for correspondence: Dr. H. Raghavendra, H. No. 101, 1st Floor, Plot No. 29, CVS Cauvery Mansion, Model Colony Beside ESI Hospital, Sunder Nagar, Hyderabad, Telangana, India. E-mail: harpanahalli.raghavendra@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neurosurgeons may find themselves in a precarious situation, while unable to secure haemostasis following tumour resection. We report intraoperative use of tranexamic acid to secure complete haemostasis as rescue measure in intracranial meningioma resection in uncontrollable bleeding.
Keywords
Difficult hemostasis
intracranial mengioma
surgical resection
tranexamic acid
INTRODUCTION
The neurosurgical patients are at increased risk of bleeding following traumatic brain injury (TBI), subarachnoid hemorrhage (SAH) as well as during intracranial surgery. The role of tranexamic acid has been evaluated in patients with TBI and SAH.[12] However, its role in intraoperative intracranial tumor bleeding has only been recently reported in a few patients.[34] We report another case of giant bilateral falcine meningioma in an adult patient, whereby an uncontrollable bleeding despite repeated attempts to achieve hemostasis was successfully managed with the use of tranexamic acid.
CASE REPORT
A 48-year-old female patient presented with abnormal behavior and urinary incontinence for 1 month. The patient was on oral telmisartan 20 mg once daily for hypertension. Magnetic resonance imaging scan of the brain revealed a large lobulated bilateral falcine homogenous contrast-enhancing mass lesion measuring 6.5 cm × 6.0 cm × 6.5 cm [Figure 1a and b], with mass effect on adjacent structures and displacement of the bilateral anterior cerebral artery. Rest of the preoperative investigations was unremarkable. The bifrontal craniotomy was carried out under general anesthesia with invasive blood pressure and central venous pressure monitoring. During tumor resection, the patient had continuous bleeding, with fluctuating hemodynamic despite using oxidized cellulose polymer. Intravascular volume was maintained with crystalloid, colloid, and packed red cells transfusion. After 3 h of surgery and following loss of almost 2 L of blood, we decided to quit. However, it appeared difficult to achieve hemostasis. The bleeding continued from the tumor bed despite repeated attempts in spite using available hemostats during surgery to control it. There prevailed a situation of uncertainty. Based on our previous experience, it was decided to administer tranexamic acid at a dose of 10 mg/kg.[5] Within 10 min of administration of tranexamic acid, there was a significant decrease in the bleeding from the tumor bed and in the next 20 min, we noted satisfactory hemostasis. This also coincided with the improvement in the hemodynamic profile of the patient and reduction of fluid to the maintenance dose. Postoperatively, the patient was shifted for elective ventilatory support and gradual reversal of neuromuscular blockade. The postoperative noncontrast computed tomography scan showed no major collections of blood in the operated field [Figure 2]. Subsequently, the patient was weaned off the ventilatory support and was discharged on the 10th postoperative day. Histopathological examination is suggestive of Grade 1 transitional meningioma.
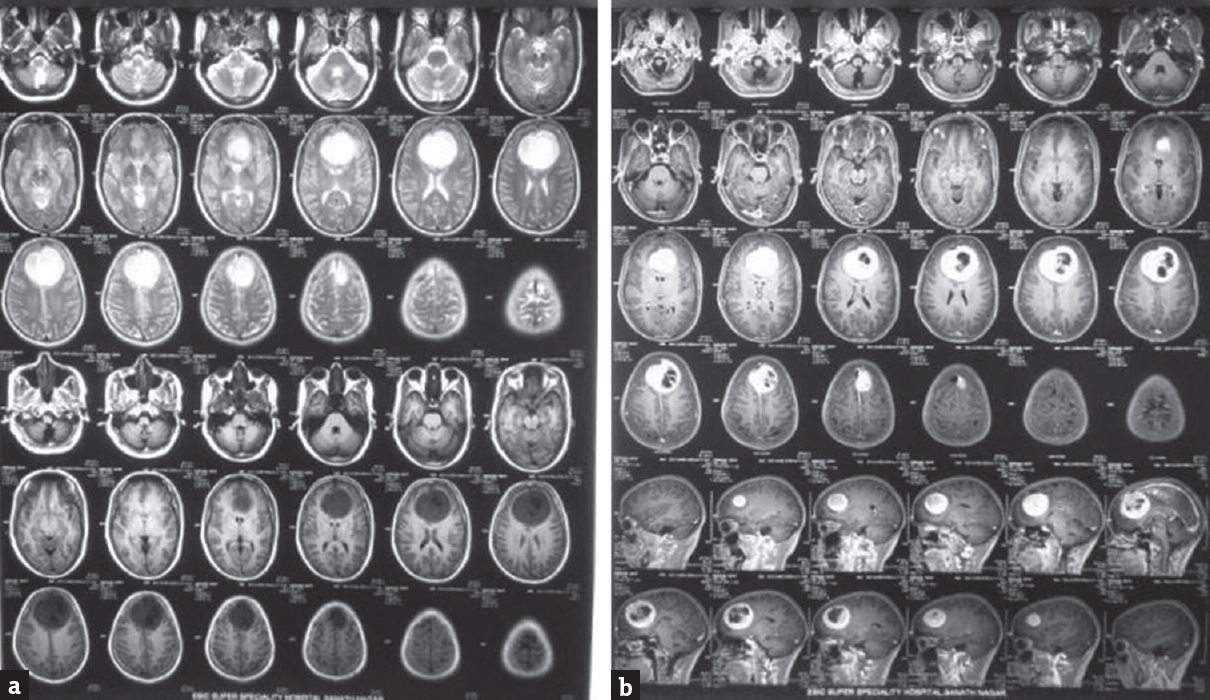
- (a and b) Magnetic resonance imaging showing large well-defined falcine lesion, hypo on T1, hyper on T2, with intensely enhancing lesion with areas of hypointensity in center of lesion
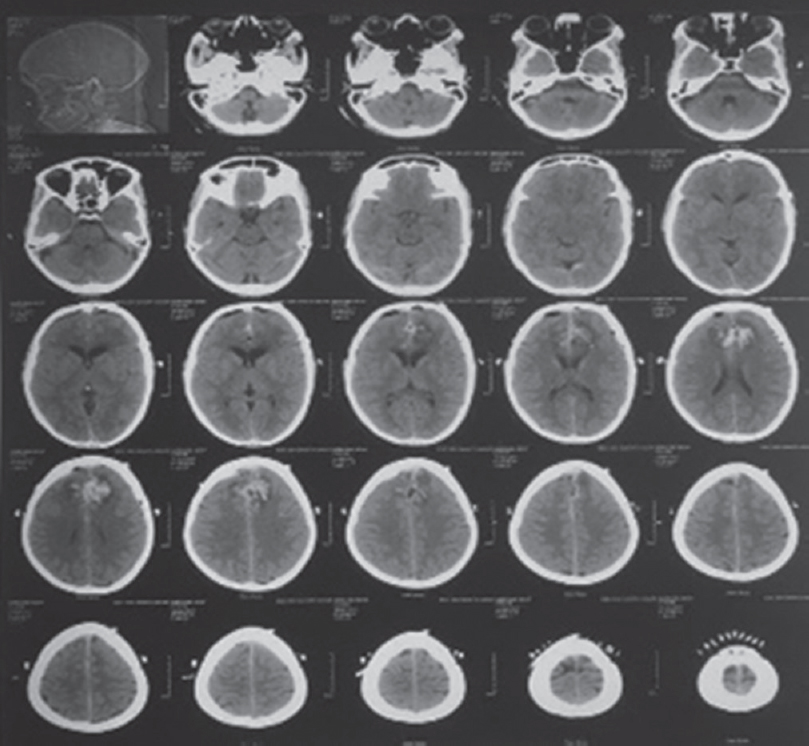
- Postoperative noncontrast computed tomography scan brain
DISCUSSION
Tranexamic acid has shown to reduce blood transfusion requirement in various surgical procedures; however, the treatment effect may vary somewhat according to the type of surgery. Its use in neurosurgical patients has been restricted to few trials of TBI and SAH due to the fear of cerebral ischemia due to thrombosis and convulsion due to gamma-aminobutyric acid antagonism.[56] Although theoretically some increased risk of cerebrovascular thrombosis might be expected, evidence from the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage-2 trial of tranexamic acid in bleeding trauma patients showed a statistically significant reduction in mortality with no increase in thromboembolic effects. Furthermore, there was a significant reduction in the risk of myocardial infarction in trauma patients who received tranexamic acid.[2] Another report suggests no evidence in increased risk of either ischemic clinical manifestation or vasospasm after tranexamic acid use for the prevention of secondary bleed in patients with subdural hematoma.[1]
The evidence for use of tranexamic acid to treat massive blood loss during intracranial surgery is weak.[4] We used this as a desperate attempt to salvage the patient as multiple blood product transfusion, and surgical efforts to stop bleeding for a prolonged period was unrewarding. The present case report supports the role of tranexamic acid in patients who have significant intraoperative bleeding with difficulty in achieving hemostasis. However, this report is only a single personal experience and event might have occurred by chance. Only a prospective randomized trial can substantiate or refute the benefit of tranexamic acid use for controlling blood loss in this subset of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. J Neurosurg. 2002;97:771-8.
- [Google Scholar]
- CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: The effect of tranexamic acid in traumatic brain injury – A nested randomised, placebo-controlled trial. Health Technol Assess. 2012;16:iii.
- [Google Scholar]
- Tranexamic acid may be beneficial for difficult hemostasis following tumor resection in neurosurgical patients: A personal experience. Asian J Neurosurg. 2017;12:309-10.
- [Google Scholar]
- Use of tranexamic acid as a rescue measure to achieve hemostasis after massive blood loss in a pediatric neurosurgical patient. J Neurosurg Anesthesiol. 2011;23:376-7.
- [Google Scholar]
- Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: A consecutive controlled clinical trial. Neurosurgery. 1981;8:158-65.
- [Google Scholar]
- Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid (A) receptor antagonistic effect. J Pharmacol Exp Ther. 2002;301:168-73.
- [Google Scholar]






