Translate this page into:
Revisiting Intraoperative 2D USG with Saline–Air Mixture as Contrast for Resection of Eloquent Area Glioma in Resource-Deficient Countries
Sushanta K. Sahoo, MCh Department of Neurosurgery, Post Graduate Institute of Medical Education & Research Madhya Marg, Sector 12, Chandigarh 160012 India drsushantsahoo@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Advanced ultrasound, intraoperative magnetic resonance imaging (MRI), neuromonitoring, and aminolevulenic acid have improved the resection and safety of eloquent area gliomas. However, availability of these modern gadgets is a major concern in resource-deficient countries. A two-dimensional ultrasonography 2D USG is cheaper, provides real-time imaging, and is already established but underutilized instrument.
Objective Here, we revisited the principles of 2D USG and used it for eloquent-area glioma surgery.
Materials and Methods Fifty-eight patients with eloquent area gliomas were operated in last 2 years with the aid of 2D USG with 6-13 MHz curvilinear probe. Preoperative diagnosis was high-grade glioma in 38 and low-grade glioma (LGG) in 20 patients. Tumors were categorized as predominantly hyperechoic (27), uniformly hyperechoic (7), mixed echogenicity (21), and cystic (3).
Results Intraoperatively, 2D USG could define the tumor margins in 46 cases. Of these, USG suggested gross total excision in 38 patients and subtotal in 8 patients. The findings matched with follow-up MRI in 34 patients who showed hyperechogenicity (predominant/uniform). Injecting saline with air in to the resection cavity and insinuating through adjacent brain parenchyma helped in detecting residual lesion in three cystic gliomas and in two LGG where the tumor cavity collapsed.
Conclusion 2D USG is a helpful tool in eloquent area glioma surgery, especially in resource-limited countries. Visualization through adjacent parenchyma and injection of saline–air mixture in to the resection cavity helped in delineating residual lesion. Extent of resection is best monitored by 2D USG when tumor appeared hyperechoic (predominant/uniform).
Keywords
intraoperative ultrasonography
eloquent area glioma
low-grade glioma
high-grade glioma
Introduction
Eloquent area glioma surgery is technically demanding. Maximal resection with minimum neurological deficit affects the long-term functional outcome. Several adjuncts such as neuronavigation, image guidance system, intraoperative magnetic resonance imaging (MRI)/computed tomogram (CT) imaging, neurophysiological monitoring, and three-dimensional ultrasonography (3D USG) have been described to improve the extent of safe resection.1 2 3 However, these are expensive tools and may not be available in resource-deficient countries. A two-dimensional USG (2D USG) is easily available and can be considered as an alternative. The major disadvantage of 2D USG is that the tumor–brain interface may not be clear in each plane. Again, the image quality may get affected due to artifacts toward the end of surgery.4 Adding a contrast along the tumor–brain interface increases the chance of picking it up even with 2D USG. In this study, we used water with air bubbles that created multiple vortices along the tumor cavity and these air bubbles within water acted as a contrast picking up the residual lesion. Again insinuating the probe through normal brain parenchyma adjacent to the corticectomy helps in maintaining the image quality. We discussed our experience of 58 cases of supratentorial eloquent area gliomas where 2D USG was used as an adjunct for achieving maximal tumor resection.
Materials and Methods
Fifty-eight patients with primary supratentorial eloquent area gliomas operated between Aug 2018 and Jan 2020 were included in this study where the tumor resection was guided by intraoperative 2DUSG (IOUSG). Patients operated for recurrent or residual lesions were excluded from this study. Patient demographic profile with clinical details was documented. All patients underwent radiological evaluation that included noncontrast CT scan and contrast-enhanced MRI in the preoperative period. Provisional categorization of tumors broadly in to benign or malignant was done on the basis of radiology. However, advanced radiological evaluation such as functional MRI and MR tractography was not performed in these patients as majority required early surgery due to raised intracranial pressure. Other intraoperative adjuncts such as 5-aminolevulenic acid (5-ALA), 3D USG, or intraoperative MRI were not available. All patients were operated under general anesthesia with IOUSG guidance to determine the extent of tumor resection. A curved phased array probe with 6-13 MHz (Sonosite, Micromax, Bothell, Washington, United Sattes) was used in all cases.
Surgical Technique
Patients were positioned so that the tumor remained perpendicular to the cortical surface so that USG probe could be easily insinuated. After positioning, incision was planned considering a wide craniotomy (tumor size + 2 cm). The USG probe was placed on normal brain parenchyma adjacent to the planned corticectomy at an angle of 60 to 90 degrees. The surgical field was divided in to four quadrants and the probe was swept sequentially in all four quadrants to visualize the tumor margin all around. Again at each quadrant images were noted in sagittal and coronal panes by tilting the probe.
Preoperative MRI was studied in detail to identify the relation of tumor with adjacent eloquent gyrus. Corticectomy was planned depending on the tumor location in relation to the central sulcus or adjacent eloquent area. When the tumor was coming up to the surface, then the corticectomy was done directly over the tumor prominence away from the eloquent gyrus. The tumor echogenicity, tumor–brain interfaces, and volume of the tumor was carefully assessed with the help of IOUSG. On the basis of echogenicity, tumors were categorized as predominantly hyperechoic or hypoechoic, uniformly hyperechoic or hypoechoic and mixed echoic. The tumor–brain interface was graded as either good (tumor and brain echogenicity can be completely differentiated) or poor (tumor and brain echogenicity are poorly differentiated) on USG. Tumor resection was started with a small corticectomy, and the USG probe was placed over the normal adjacent parenchyma in all possible quadrants. At frequent intervals, the tumor cavity was injected with a mixture of air and saline to objectively assess the extent of tumor resection. A 20 mL syringe was partly filled with saline and when injected into the tumor cavity, the mixture of air and water thus acted as a separate layer just like a contrast media delineating the resection margin. If any residual tumor detected at any quadrant, resection under operating microscope was attempted till reaching up to the margin.
Detecting End-Point of Tumor Resection
Under operating microscope completion of tumor excision was considered as reaching up to the normal brain (yellowish white, relatively nonsuckable). At this point, USG probe was placed at the adjacent brain near margins of resection in all possible four quadrants as described above. Then the cavity was filled with water and air bubbles. Thereby two separate hyperechoic rims at the margin of tumor resection created, inner one due to air bubbles and outer one due to blood. Absence of any tumor echogenicity outside these hyperechoic rims confirms ultrasonic completion of tumor resection. At 3 months, postoperative contrast-enhanced MRI was obtained in each case. The postoperative MRI was evaluated independently by neuroradiologist to determine the extent of resection.
Results
The male to female ratio was 1:2 and their age ranged from 18 to 66 years. Radiological (MRI) diagnosis of high-grade glioma (HGG) was made in 38 and low-grade glioma (LGG) in 20 patients (Figs. 1A, 2A–B, 3A–C). The average tumor dimension was 4.8 × 3.6 × 2.2 cm in axial coronal and sagittal plane. There were 32 lesions close to the sensory motor area, 5 were insular glioma, 3 were on the speech area, 3 in the thalamic region, 12 related to the angular gyrus, and 3 on the occipital region close to the visual cortex. At presentation, 3 patients had hemiparesis, 9 had monoparesis, 2 had aphasia, and 2 had parietal lobe features such as apraxia and Gerstmann syndrome. In addition, headache and seizure were present in 49 patients, warranting early surgery.
-
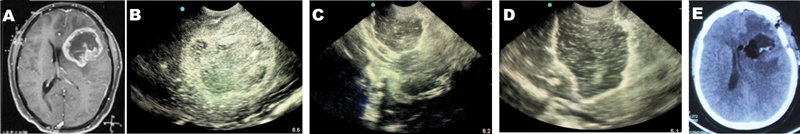
Fig. 1 (A) Preoperative magnetic resonance imaging showing heterogenous contrast-enhanced lesion in the left frontal operculoinsular region suggestive of a high-grade glioma. (B) Intraoperative ultrasonography showing mixed echogenic lesion with a hyperechoic rim. (C) After partial resection, the tumor cavity and the residual lesion are visualized. Saline with air bubbles filled the entire resection cavity after complete resection of the lesion. Note the hyperechoic rim formed at the periphery of the cavity due to blood clot and air bubbles. (D) Postoperative computed tomography (CT) scan showing resection of the lesion. (E) Immediate post operative CT showing complete resection of the lesion.
Fig. 1 (A) Preoperative magnetic resonance imaging showing heterogenous contrast-enhanced lesion in the left frontal operculoinsular region suggestive of a high-grade glioma. (B) Intraoperative ultrasonography showing mixed echogenic lesion with a hyperechoic rim. (C) After partial resection, the tumor cavity and the residual lesion are visualized. Saline with air bubbles filled the entire resection cavity after complete resection of the lesion. Note the hyperechoic rim formed at the periphery of the cavity due to blood clot and air bubbles. (D) Postoperative computed tomography (CT) scan showing resection of the lesion. (E) Immediate post operative CT showing complete resection of the lesion.
-
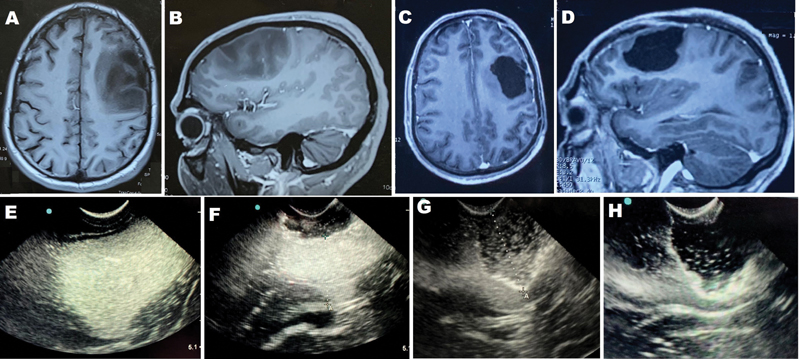
Fig. 2 (A and B): Preoperative contrast-enhanced magnetic resonance imaging (MRIs) (axial and sagittal) showing left posterior frontal nonenhanced hypointense lesion suggestive of low-grade glioma. (C and D) Postoperative axial and sagittal MRIs showing complete resection of the lesion. (E–H) Intra operative ultrasound images. Tumor appears uniformly hyperechoic with good tumor–brain interface (E). After partial resection residual lesion visualized and margin of lesion well differentiated (F–G). No tumor echogenicity seen beyond the hyperechoic rim suggestive of complete removal of tumor (H).
Fig. 2 (A and B): Preoperative contrast-enhanced magnetic resonance imaging (MRIs) (axial and sagittal) showing left posterior frontal nonenhanced hypointense lesion suggestive of low-grade glioma. (C and D) Postoperative axial and sagittal MRIs showing complete resection of the lesion. (E–H) Intra operative ultrasound images. Tumor appears uniformly hyperechoic with good tumor–brain interface (E). After partial resection residual lesion visualized and margin of lesion well differentiated (F–G). No tumor echogenicity seen beyond the hyperechoic rim suggestive of complete removal of tumor (H).
-
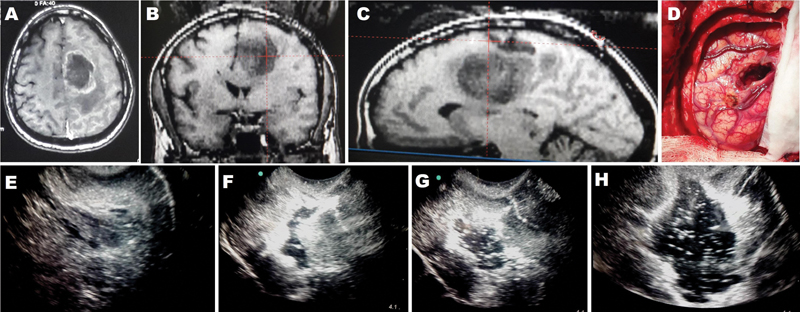
Fig. 3 (A–C) Preoperative magnetic resonance imaging (axial, coronal, sagittal) showing hypointense left posterior frontal subcortical lesion. (D) Intraoperative image showing anteriorly paced corticectomy in the operative field. (E) Intraoperative 2D ultrasonography (IOUSG) image showing mixed echogenic lesion with good brain–tumor interface. (F) IOUSG image showing collapse of the resection cavity with ill-defined residual lesion. (G) IOUSG image showing re-expansion of the resection cavity after injection of saline with air bubbles. (H) IOUSG image showing further progression of lesion and the tumor–brain interface is visualized clearly.
Fig. 3 (A–C) Preoperative magnetic resonance imaging (axial, coronal, sagittal) showing hypointense left posterior frontal subcortical lesion. (D) Intraoperative image showing anteriorly paced corticectomy in the operative field. (E) Intraoperative 2D ultrasonography (IOUSG) image showing mixed echogenic lesion with good brain–tumor interface. (F) IOUSG image showing collapse of the resection cavity with ill-defined residual lesion. (G) IOUSG image showing re-expansion of the resection cavity after injection of saline with air bubbles. (H) IOUSG image showing further progression of lesion and the tumor–brain interface is visualized clearly.
Tumor Appearance on USG
In all cases, the USG probe could be insinuated through the main craniotomy corridor (Fig. 3D). In all patients, the tumor could be localized clearly and differentiated from normal brain and edema (Figs. 1B, 2E, 3E) Out of 58 cases, tumor was predominantly hyperechoic in 27, uniformly hyperechoic in 7, mixed echogenic in 21, and hyperechoic rim in 3 cases. The tumor–brain interface was good in 46 patients (34 HGG, 12 LGG) and poor in 12 patients (4 HGG, 8 LGG).
In 34 cases of HGG that showed good brain–tumor interface on IOUSG, the tool aided in determining extent of resection. Of these 3 cases of cystic glioma, the cyst cavity collapsed during surgery. After injection of saline with air bubbles, the resection cavity re-expanded and residual lesion was better delineated. At the end of resection, well-defined hyperechoic rim was detected in all cases of HGG (Fig. 1D).
In 12 cases of LGG, the brain–tumor interface was good. In 7 of them, the tumor was homogenously hyperechoic and tumor–brain interface was maintained till the end of resection. (Fig. 2E–H). In two patients, the wall of resection cavity fell on each other after partial resection of the lesion and identification of residual was difficult. When saline with air bubbles was injected in to the resection cavity with a 20 mL syringe, the resection margin as well as the residual lesion could be visualized better (Fig. 3F–G).
Extent of Resection and Clinical Outcome
Extent of tumor resection using IOUSG was assessed in 46 patients where the tumor–brain interface was good. In remaining 12 patients, the tumor–brain interface was poorly delineated on IOUSG and the end-point of resection was difficult to assess. In these patients, complete resection was not attempted and therefore excluded from the final image analysis. Of these 46 patients, gross total resection (GTR) was achieved in 38 (31 HGG and 7 LGG) cases as per IOUSG criteria (Figs. 1D, 2 H, 3H). In 8 cases (3 thalamic tumor and 5 insular glioma), part of tumor close to the internal capsule was left to avoid postoperative focal neurological deficit.
Follow-up MRI matched with IOUSG findings in 34 (73.91%) cases (29 with GTE and 5 with subtotal excision) (Figs. 1A, E, 2A–D). In 9 out of 38 (23.68%) cases where IOUSG confirmed GTR, residual lesion was detected on follow-up MRI. In all these cases, the tumor was mixed echoic appearance on IOUSG. Similarly, there was significant discordance between IOUSG findings and follow-up MRI in 3 out of 8 (37.5%) cases where subtotal excision was done. MRI showed considerably large residue in all these 3 cases where only marginal tissue was left on the basis of IOUSG findings.
Six patients of 38 where ultrasonically GTR was achieved developed new onset neurological deficits (hemiparesis in 4 and aphasia in 2) in the postoperative period. Two of these patients improved completely and one showed partial improvement at 6 months follow-up. The remaining 3 patients had permanent deficits. One patient with preoperative hemiparesis improved after GTR of the lesion. None of the patients who underwent subtotal resection developed additional deficits.
Discussion
Maximal safe resection is desirable in intraparenchymal lesions that may offer good neurological outcome and improved quality of life.1 2 However, complete excision at times is difficult and may not be possible in lesions located at eloquent brain areas. With improvement in technology, various adjuncts such as intraoperative MRI, neuronavigation, fluorescence, and 5-ALA have been added to neurosurgery armamentarium to improve the extent of glioma resection. Recently, 3D USG with facility for contrast injection is gaining popularity and is considered superior because of its real-time imaging. The image resolution is better in 3D USG and tissue differentiation is superior.3 5 6 However, all these modalities are expensive and may not be available in resource-limited countries. Although 5-ALA helps to delineate the residual tumor during surgery, but it is not widely available and expensive. Only a few neurosurgical centers have the facility for intraoperative MRI and 3D USG due to financial constraints. Although these adjuncts facilitate the extent and safety of intraparenchymal tumor removal but in their absence, a simple 2D USG can be used for intraoperative tumor localization. The patient and caregivers must be counseled about all these advanced adjuncts and their availability issues. Proper explained consent should be obtained prior to surgery for standalone use of 2D USG to avoid litigation in future. A 2D USG is easily available yet underutilized instrument for intraoperative use in glioma surgery. This provides real-time imaging of intraparenchymal lesions and can be used as a cheaper but reliable alternative. The use of 2D USG in glioma surgery has been described in the past with good outcome.4 7
The intraoperative USG image quality is mostly user dependent. Proper image acquisition and interpretation are necessary throughout the surgical procedure. The gap between the lesion and the probe has to be minimum to avoid artifacts. The gap between the brain and the USG probe has to be filled with an ideal medium with similar acoustic properties of the brain.8 9 However, sterile gels may be used as a coupling agent to reduce air artifacts. A phase array probe provides a fan-shaped image covering a wide area at the depth and a narrow sector on the surface.8 Therefore, we prefer a wide craniotomy in relation to the tumor, where probe was placed over normal parenchyma adjacent to the corticectomy. Insinuating the USG through normal brain parenchyma may avoid use of additional layer of coupling agent. The concept of keeping the resection cavity vertical and periodically filling it with saline is an established technique to avoid air artifacts.4 However, probe placement over normal brain away from the corticectomy site and simultaneously filling the cavity with saline reduce the possibility of image distortion due to air artifact.
It is of paramount importance to preserve the adjacent white fiber tracts in eloquent area glioma surgery. Preoperative functional MRI or MRI with tractography helps to identify the relationship of these eloquent structures adjacent to the tumor. Intraoperative use of neuro-navigation, fiber tracking, and awake craniotomy with neurophysiological monitoring are the ideal methods for intraoperative identification of displaced white fiber tracts. In conjunction with an IOUSG, maximal safe resection is achievable.3 5 6 2D IOUSG alone is unlikely to yield equivalent results. Nevertheless, it still is an important adjunct in resource-deficient centers. The tumor–brain interface has to be observed carefully throughout the surgery in order to avoid inadvertent injury in cases of eloquent area gliomas. The image quality of the intraoperative USG largely depends on the probe placement. Larger craniotomy is needed for visualizing the entire operative field with a standard 2D USG phase array probe. Sequential swiping of USG probe in all possible four quadrants enables the surgeon to visualize the tumor margin all around. Imaging through multiple planes thereby helps the surgeon to assess the tumor in three dimensions even with a 2D USG.
With progression of surgery, the irregular margins of resection cavity with blood products produce hyperechoic artifacts.8 At times, the tumor cavity may collapse distorting the image quality, and the risk of injury to adjacent eloquent brain thus increases. 3D USG with contrast at this point differentiates the residual lesion owing to its hypervascularity.3 6 Here, we have injected saline with air that re-expanded the tumor cavity. The vortices produced by this air mixture acted as a contrast that aligned along the tumor cavity differentiating the residual lesion from normal brain parenchyma. A smaller size high-frequency probe is also helpful in delineating the tumor margins.10 But these are expensive and may not be available in resource-scarce health-care systems.
Because of hypercellularity, most of brain tumors appear hyperechoic as compared to normal brain parenchyma.11 Tumor margins were better differentiated when appeared uniformly hyperechoic. In our experience, these lesions form a specific subgroup where the ultrasonic image was maintained even toward the end of surgery. In all seven cases in our series where the tumor was uniformly hyperechoic, intraoperative findings matched well with the follow-up MRI. When the tumor was mixed echoic, the extent resection determined by IOUSG may not match accurately with follow-up MRI as seen in nine of our patients. In these cases, the tumor could be well delineated in the beginning. But with the progression of resection as the tumor tissue becomes thinner, the differentiation of brain and tumor echogenicity was not uniform in all four quadrants. In these cases, the tumor–brain interface was more difficult to appreciate at the end of surgery. Again two of them had significant peritumoral brain edema. As the tumor echogenicity was not uniform, it further adds to the dilemma in identifying its edges. Therefore, we believe the tumor echogenicity pattern on IOUSG largely affects the extent of resection.
In three cases where planned subtotal resection was done, the residual lesion appeared larger in the follow-up MRI. At the end of surgery, the residual lesion may not have uniform thickness and we have assessed the residual tumor (all four quadrants) in two dimensions only. This probably results in a false impression of apparent small residue on IOUSG. Intracavitary linear probe USG would have been better to estimate the residual tumor in planned subtotal resection where all corners of tumor cavity can be assessed in multiple planes.
In some series, the extent of tumor resection as determined by IOUSG correlated strongly with postoperative MRI.7 12 However, some of these patients were operated in awake condition or with intraoperative use of ALA for tumor localization and resection.12 In another meta-analysis, where the extent of tumor resection was assessed, the concordance rate between IOUSG and MRI was 82%.13 Yet again intraoperative image guidance system and ALA-induced fluorescence were used in many of the studies analyzed. In another series, the IOUSG correctly identified the histopathological tumor margin in 66 out of 79 cases (83.5%).14 Identification and resection of the residual tumor close to the eloquent region are difficult without the aid of intraoperative image guidance system and awake mapping.
Limitations
A 2D USG is helpful in locating the intraparenchymal brain lesions. Loss of tumor–brain interface with progression of surgery is a major concern with 2D USG. For deep seated lesions located close to white fiber tracts, stand-alone IOUSG may not yield desired tumor resection. Neuronavigation and neuromonitoring if available should be used in addition.
Conclusion
IOUSG with a wide craniotomy is a reasonably good tool to safely resect gliomas located in eloquent areas. The use of saline with air mixture as a contrast aids in resection especially the cystic ones and cases where the tumor walls tend to collapse. The extent of resection is comparable with follow-up MRI, especially in lesions that appeared predominantly hyperechoic or uniformly hyperechoic. However, 2D USG may not give accurate idea of the volume of residual lesion when subtotal excision is planned.
Conflict of Interest
None declared.
References
- A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198.
- [Google Scholar]
- Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576. , discussion 564–576
- [Google Scholar]
- Fusion imaging for intra-operative ultrasound-based navigation in neurosurgery. J Ultrasound. 2014;17(3):243-251.
- [Google Scholar]
- Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery. 2002;51(2):402-411. , discussion 411–412
- [Google Scholar]
- The use of ultrasound in intracranial tumor surgery. Acta Neurochir (Wien). 2016;158(6):1179-1185.
- [Google Scholar]
- Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir (Wien). 2006;148(3):235-253. , discussion 253
- [Google Scholar]
- Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: a comparative study with magnetic resonance imaging. J Neurosurg. 1996;84(5):737-741.
- [Google Scholar]
- Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir (Wien). 2013;155(6):973-980.
- [Google Scholar]
- Animal study assessing safety of an acoustic coupling fluid that holds the potential to avoid surgically induced artifacts in 3D ultrasound guided operations. BMC Med Imaging. 2014;14:11.
- [Google Scholar]
- Applications of ultrasound in the resection of brain tumors. J Neuroimaging. 2017;27(1):5-15.
- [Google Scholar]
- A practical grading system of ultrasonographic visibility for intracerebral lesions. Acta Neurochir (Wien). 2013;155(12):2293-2298.
- [Google Scholar]
- Extent of glioma resection on intraoperative ultrasound correlates well with postoperative MRI results. J Surg Oncol. 2019;2(4):1-7.
- [Google Scholar]
- Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255-263.
- [Google Scholar]
- Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours–a comparative study with computed tomography and histopathology. Acta Neurochir (Wien). 2003;145(9):743-748. , discussion 748
- [Google Scholar]






