Translate this page into:
Assessment of Optic Nerve Sheath Diameter and Its Postoperative Regression among Patients Undergoing Brain Tumor Resection in a Tertiary Care Center
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction Bedside measurement of optic nerve sheath diameter (ONSD) using ultrasonography (USG) is a useful method for detecting raised intracranial pressure (ICP). The primary and main objective of this study is to estimate ONSD among patients with brain tumor and its regression post tumor resection to assess the correlation as well as diagnostic accuracy of the same.
Materials and Methods This prospective observational study was performed in a tertiary health care center over a span of 3 months on 68 adults of either sex, out of which 30 were nonneurosurgical patients, taken as control group. Rest 38 were neurosurgical patients posted for brain tumor resection. Normal ONSD in our population was determined by calculating average ONSD using transorbital USG in individuals of control group. ONSD in neurosurgical patients taken as case group was recorded before surgery, intraoperatively immediately post tumor resection, as well as 12 and 24 hours post surgery. These values were analyzed to see the correlation of ONSD with tumor resection.
Results The mean (±standard deviation) binocular ONSD in our population was 4.28 ± 0.28 mm. The mean preoperative binocular ONSD in cases using transorbital USG came out to be 5.43 ± 0.37 mm with 88.23% sensitivity and 100% specificity. Postoperatively, transorbital ONSD showed significant regression at 12 and 24 hours as compared with preoperative values (p-value < 0.05).
Conclusion Transorbital ultrasonographic measurement of ONSD could be considered as an indirect indicator of ICP in neurosurgical patients perioperatively. The technique is quick to perform at bedside, feasible in critical patients, and without any harmful effects.
Keywords
transorbital optic nerve sheath diameter
ultrasonography
intracranial pressure
neurosurgery
tumors
Introduction
The term “brain tumors” refers to a mixed group of neoplasms originating from intracranial tissues and the meninges with degrees of malignancy ranging from benign to aggressive. The majority of brain tumors in adults are supratentorial in location. Gliomas, meningiomas, and pituitary adenomas are the most common.1
Raised intracranial pressure (ICP) is a very common problem in neurosurgical and neurological practice. It can arise as a result of intracranial mass lesions, disorder/disruptions of cerebrospinal fluid (CSF) circulation, and more diffuse intracranial pathological diseases such as tumor, trauma, stroke, hydrocephalus, and hypoxia.2 Avoidance of brain insults secondary to raised ICP is one of the key components in the perioperative management of neurosurgical patients.
Monitoring of ICP therefore becomes necessary in patients presenting with space-occupying lesion. Invasive methods such as ventriculostomy prove to be the most accurate at measuring ICP and are considered to be gold standard, but they are prone to a variety of complications including infection, hemorrhage, and neurological deficits. Moreover, invasive techniques may not be ideal in many resource-limited settings.
In recent years, several noninvasive techniques have been developed to monitor ICP. Measurement of optic nerve sheath diameter (ONSD) by ultrasonography (USG) technique is one of such techniques and an increase in ONSD has been suggested as a possible indicator of intracranial hypertension. The use of bedside ocular USG in measuring of ONSD can be a useful method for detecting raised ICP. It has the advantage of being a noninvasive, portable, and easily performed approach. The technique is cheap and efficient also.3
The optic nerve being a part of the central nervous system is surrounded by subarachnoid space and the pressure changes in intracranial compartment is transmitted directly into this space. An increase in ICP leads to distension of the infraorbital part of the sheath. Resolution of the ONSD size is commonly thought to be a good and reliable clinical indicator of decreased ICP post surgery.
Although the relationship between ICP and ONSD has been described by several authors with varying accuracy, but there has been scarcity of literature in relation to ONSD size and its regression after the resection of brain tumors in patients. Therefore, this study was aimed and designed to estimate the size of ONSD as well as its regression in patients of brain tumor surgery to assess the correlation as well as diagnostic accuracy of the same.
Materials and Methods
This prospective, observational study was conducted on 68 adults after approval from the ethical review board and clinical trial registration (CTRI/2021/01/030744) over a period of 3 months.
Out of these 68 individuals, 30 were patients posted for nonneurosurgical procedures under general anesthesia, aged between 15 and 55 years, of either sex, taken as control group to determine the average ONSD in our population, and measured using transorbital USG after induction of anesthesia. Rest 38 patients of either sex, aged between 15 and 55 years, with American Society of Anesthesiologists (ASA) physical status score 1 to 3, were enrolled as cases for the study over a period of 5 months. These cases were admitted for elective resection of space-occupying lesion under general anesthesia with features suggestive of raised ICP radiologically (narrowing of ventricles and cisterns, fading of the brain sulci, mass effect, midline shift, etc.). Patients with ASA status 4 to 5, Glasgow Coma score <15, optic nerve diseases such as optic neuritis, optic nerve arachnoid cyst, anterior orbital mass, known orbital injury or prior ocular surgery, and hyperthyroidism were excluded from the study.
The informed written and oral consent was signed by all the included participants prior to the study. General anesthesia was given to all patients as per institutional protocol. Patients' eyes were scanned in supine position using a high-resolution 7-MHz linear array transducer (Hitachi Medical Corporation, Tokyo, Japan) on closed eyelids. The structure of the eyes was visualized to align with the optic nerve directly opposite the probe with the ONSD width perpendicular to the vertical axis of the scanning plane. ONSD bilaterally was measured 3 mm behind the globe and an average of three readings from each eye was taken as shown in Fig. 1. Transorbital ONSD value greater than 5 mm was considered as indicative of raised ICP.4
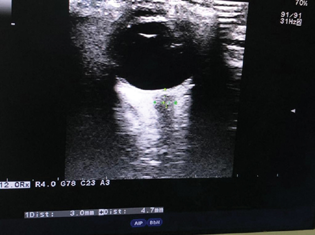
-
Fig. 1 Transorbital measurement of optic nerve sheath diameter using ultrasonography.
Fig. 1 Transorbital measurement of optic nerve sheath diameter using ultrasonography.
ONSD measurements were done at T1, T2, T3, and T4 intervals.
-
T1: After induction of anesthesia but before the starting of surgical procedure.
-
T2: At the completion of surgery before extubation.
-
T3: 12 hours of post tumor resection.
-
T4: 24 hours of post tumor resection.
These values were analyzed to see the correlation of ONSD with tumor resection. Transorbital USG was performed by a single senior neuroanesthetist in all patients to remove observer bias. Patients where satisfactory tumor resection could not be achieved or were complicated due to iatrogenic surgical bleeding or patients who could not be extubated within 12 hours of completion of surgery were not included in final analysis.
Statistical Methods
The sample size estimation was performed in accordance with the effect size (0.5) of a study comparing the ONSD using magnetic resonance imaging (MRI) in patients before and after the shunt operation.5 A sample size of 34 cases with α = 0.05 and 80% power was calculated. Total of 38 cases were enrolled to allow for 10% loss from analysis.
All quantitative variables were checked for normal distribution within each category of explanatory variable by using visual inspection of histograms and normality Q-Q plots. Shapiro–Wilk test was also conducted to assess normal distribution. Shapiro–Wilk test p-value of > 0.05 was considered as normal distribution.
The independent sample t-test was used to compare mean values between study groups for normally distributed quantitative parameters. The chi-squared test was used to compare categorical outcomes between study groups. A p-value < 0.05 was considered as statistically significant. For statistical analysis Statistical Package for the Social Sciences, version 22 (IBM SPSS, United States) was used.
Results
Thirty-eight patients were enrolled for the study; however, data from four patients were not analyzed (Fig. 2: consort flow diagram).
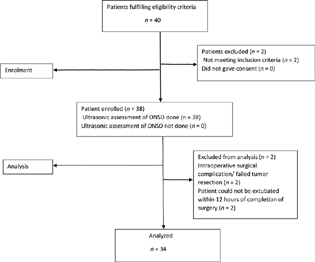
-
Fig. 2 Consort flow diagram showing the flow of participants through each stage of the study. ONSD, optic nerve sheath diameter.
Fig. 2 Consort flow diagram showing the flow of participants through each stage of the study. ONSD, optic nerve sheath diameter.
The cases and control were comparable in terms of demographic parameters (Table 1). Preoperative transorbital ONSD came out to be 88.23% sensitive and 100% specific (Table 2). The binocular ONSD mean (± standard deviation [SD]) was found to be 4.28 ± 0.28 mm in control group; however, the mean preoperative binocular ONSD in cases was 5.43 ± 0.37 mm. This difference was statistically significant (p-value < 0.001) as shown in Table 3. Mean (SD) binocular ONSD at T2 was 5.19 ± 0.72 mm and at 12 and 24 hours post tumor resection was 4.98 ± 0.47 and 4.76 ± 0.63 mm, respectively. Taking preoperative time (T1) binocular ONSD as baseline, the mean difference was statistically significant at 12 and 24 hours' time (p-value < 0.05; Table 4).
|
Parameter |
Groups |
p-Value |
|
|---|---|---|---|
|
Controls (n = 30) |
Cases (n = 34) |
||
|
Age: mean ± SD |
46.23 ± 6.22 |
43.80 ± 9.65 |
0.10 |
|
Gender: n (%) |
|||
|
Male |
14 (45%) |
16 (53%) |
1.00 |
|
Female |
16 (55%) |
18 (47%) |
|
|
ASA grade |
|||
|
ASA 1 |
20 |
8 |
|
|
ASA 2 |
10 |
12 |
|
|
ASA 3 |
0 |
10 |
|
Abbreviations: ASA, American Society of Anesthesiologists (physical status); SD, standard deviation.
|
Patients enrolled with radiological findings of raised ICP |
Mean binocular ONSD <5 mm using transorbital USG |
Mean binocular ONSD >5 mm using transorbital USG |
Sensitivity (%) |
Specificity (%) |
|---|---|---|---|---|
|
34 |
4 |
30 |
88.23 |
100 |
Abbreviations: ICP, intracranial pressure; ONSD, optic nerve sheath diameter; USG, ultrasonography.
|
Parameter |
Controls (n = 30) |
Cases (n = 34) |
p-Value |
|---|---|---|---|
|
Preoperative ONSD: right eye (mean ± SD) |
4.33 ± 0.30 |
5.40 ± 0.40 |
<0.001 |
|
Preoperative ONSD: left eye (mean ± SD) |
4.22 ± 0.27 |
5.45 ± 0.36 |
<0.001 |
|
Preoperative ONSD: binocular (mean ± SD) |
4.28 ± 0.28 |
5.43 ± 0.37 |
<0.001 |
Abbreviations: ONSD, optic nerve sheath diameter; SD standard deviation.
|
Time |
Mean ± SD |
Mean difference |
95% confidence Interval for mean |
p-Value |
|
|---|---|---|---|---|---|
|
Lower bound |
Upper bound |
||||
|
ONSD (right eye) |
|||||
|
Preoperative (T1) |
5.40 ± 0.40 |
||||
|
Postoperative (T2) |
5.14 ± 0.71 |
0.260 |
–0.01 |
0.53 |
0.067 |
|
At 12 hours (T3) |
4.91 ± 0.79 |
0.490 |
0.18 |
0.79 |
0.002 |
|
At 24 hours (T4) |
4.85 ± 0.85 |
0.550 |
0.22 |
0.87 |
0.001 |
|
ONSD (left eye) |
|||||
|
Preoperative (T1) |
5.45 ± 0.36 |
||||
|
Postoperative (T2) |
5.24 ± 0.75 |
0.210 |
–0.07 |
0.49 |
0.145 |
|
At 12 hours (T3) |
5.05 ± 0.39 |
0.400 |
0.21 |
0.58 |
<0.001 |
|
At 24 hours (T4) |
4.68 ± 0.58 |
0.770 |
0.53 |
1.00 |
<0.001 |
|
ONSD (binocular) |
|||||
|
Preoperative (T1) |
5.43 ± 0.37 |
||||
|
Postoperative (T2) |
5.19 ± 0.72 |
0.240 |
–0.03 |
0.51 |
0.088 |
|
At 12 hours (T3) |
4.98 ± 0.47 |
0.450 |
0.24 |
0.65 |
0.001 |
|
At 24 hours (T4) |
4.76 ± 0.63 |
0.670 |
0.41 |
0.92 |
<0.001 |
Abbreviations: ONSD, optic nerve sheath diameter; SD, standard deviation.
Discussion
The cranium is a rigid compartment consisting of the brain, blood, and CSF. The contents of the cranium are in a condition of volume balance, with any change in one component's volume being compensated by a change in the other's volume. When these compensating mechanisms become exhausted, ICP rises. Intracranial hypertension is an important cause of mortality and morbidity in neurosurgical patients. Monitoring of ICP and timely intervention of raised ICP therefore are essential components in management of neurosurgical patients.
Although considered to be gold standard, invasive ICP monitoring techniques may not be ideal in resource-limited settings. In recent years, noninvasive methods of ICP monitoring have been proposed and ultrasonographic measurement of ONSD is one such technique that can be effectively used in postoperative neurosurgical patients.
Thirty elective nonneurosurgical patients were chosen as control to determine the average value of ONSD in our population, which came out to be 4.28 mm. Almost similar values were reported by Shirodkar et al, in which the average ONSD was 4.6 mm and 4.8 mm in females and males, respectively.6 In our study, the binocular mean preoperative ONSD among cases was 5.43 mm, which was significantly higher in comparison to value of ONSD in control group (p-value < 0.001). We considered values above 5 mm as an indicator of raised ICP.4 Rajajee et al, however, reported that ONSD > 4.8 mm has the greatest accuracy to identify ICP > 20 mm Hg in a heterogeneous group of patients with acute brain injury.7 Dubourg et al, in systematic review and meta-analysis, concluded that ONSD > 5.00 to 5.70 mm has a raised ICP of > 20 mm Hg.8 The findings were in agreement to our study. All the patients in our study had features of raised ICP preoperatively, as evident radiologically. Transorbital ultrasonic assessment of ONSD in these patients revealed a mean ONSD > 5 mm before surgical resection, with 88.23% sensitivity and 100% specificity. Multiple studies suggested a positive linear relationship between ONSD and ICP, that is, an immediate change in ONSD occurs with a change in ICP.9 10 11 In our study, there was no significant change in ONSD immediately after tumor resection; however, the ONSD observed at 12 and 24 hours postoperatively reflected significant resolution in diameter, which correlated with clinical improvement. These results were in line with Singhal et al, who reported a significant reduction in ONSD on MRI post endoscopic third ventriculostomy and tumor resection.5 However, their sample size was small as compared with our study; also, bedside ultrasonic assessment of ICP using ONSD is much feasible as compared with the whole process of MRI for a critical patient. Similarly, Watanabe et al in their study observed a significant reduction in ONSD after surgery.12 In our study, the statistically significant changes in ONSD were appreciable after 12 and 24 hours of surgical decompression, which was in contrast to the Watanabe et al study.12 We assume that this difference may be due to the change in the dynamics of subarachnoid space and ICP after tumor resection and surgical handling.
There are certain limitations of our study. We did not take into account the size of the tumors, which could have possibly impacted the size of ONSD. The study was conducted only in supratentorial tumors. The dynamics of regression of ONSD is assumed to be different in supratentorial and infratentorial tumors, the infratentorial compartment being a rigid space and the mean values of ICP being supposedly higher in the posterior fossa.13 Additionally, study was done on mixed group of a supratentorial mass (including meningioma, glioma, and pituitary adenoma). A better approach would have been to compare the regression of ONSD in homogeneous group of brain tumors. We did not correlate the regression of ONSD with ICP monitored with invasive monitor. We are of the view that monitoring of ICP invasively may not be always feasible in all postoperative neurosurgical patients in resource-limited setting.
Conclusion
The use of bedside ocular USG and serial ONSD measures to evaluate ICP after tumor removal is a convenient and reliable approach. It should, however, be utilized in conjunction with traditional radiological criteria to aid in management.
Conflict of Interest
None declared.
Funding None.
References
- Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: a systematic review. Neurocrit Care. 2007;6(2):104-112.
- [Google Scholar]
- Measurement and management of increased intracranial pressure. Open Crit Care Med J. 2013;6(1):56-65.
- [Google Scholar]
- Optic nerve sheath diameter measurement for predicting raised intracranial pressure in adult patients with severe traumatic brain injury: a meta-analysis. J Crit Care. 2020;56:182-187.
- [Google Scholar]
- Does optic nerve sheath diameter on MRI decrease with clinically improved pediatric hydrocephalus? Childs Nerv Syst. 2013;29(2):269-274.
- [Google Scholar]
- Optic nerve sheath diameter as a marker for evaluation and prognostication of intracranial pressure in Indian patients: an observational study. Indian J Crit Care Med. 2014;18(11):728-734.
- [Google Scholar]
- Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15(3):506-515.
- [Google Scholar]
- Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068.
- [Google Scholar]
- The correlation between endocranial pressure and optic nerve diameter: an ultrasonographic study.Ophthalmic Echography. Springer Nature Switzerland AG; 1987. p. :603-606. In:
- [Google Scholar]
- Echographic measurement of the optic nerve in patients with intracranial hypertension. Neurochirurgia (Stuttg). 1987;30(2):53-55.
- [Google Scholar]
- CSF dynamic parameters and changes of optic nerve diameters measured by standardized echography.Ophthalmic Echography 13. Springer, Dordrecht; 1993. p. :101-109. In eds.
- [Google Scholar]
- Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg. 2008;109(2):255-258.
- [Google Scholar]
- Characterisation of supra- and infratentorial ICP profiles. Acta Neurochir Suppl (Wien). 2016;122:37-40.
- [Google Scholar]







