Translate this page into:
Related Factors and Predictors of Cognitive Dysfunction in Chronic Kidney Disease on Maintenance Hemodialysis in Nigeria
Address for correspondence: Dr. Lukman Femi Owolabi, Department of Neurology, Aminu Kano Teaching Hospital, Bayero University, PMB 3452, Kano, Nigeria. E-mail: drlukmanowolabi@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Previous studies suggest a high frequency of cognitive impairment (CI) in persons with chronic kidney disease (CKD); however, factors associated with CI and predictors of CI in persons with CKD remain largely unclear. The aim of this study was to determine the factors associated with CI and predictors of CI in CKD patients on maintenance hemodialysis.
Materials and Methods:
The first stage of the study included recruitment of 100 apparently healthy participants aimed at determining the reference values. The second stage of the study included eighty CKD patients on maintenance hemodialysis. The iron psychology (FEPSY) was used to assess the memory, psychomotor speed, concentration, and attention using simple auditory reaction time (ART) and visual reaction time (VRT) tasks, recognition memory tests (RMT), finger tapping task (FTT), and binary choice task (BCT).
Results:
Using normative values generated in this study, 41 (51.3%) and 43 (53.8%) CKD patients had abnormal scores on ART dominant (D) and nondominant (ND) sides, respectively. Forty (50%) and 42 (52.5%) patients had abnormal scores on VRT D and ND sides, respectively. Twenty-one (26.3%) and 68 (85%) had abnormal scores on BCT and computer-assisted visual scanning task, respectively. Sixty-four (80%) and 65 (81.3%) had abnormal scores on RMT (words) and RMT, respectively. Fifty-two (65%) and 48 (60%) patients had abnormal scores on D and ND sides of (FTT), respectively. Factors associated with psychomotor speed impairment were duration of CKD from diagnosis (P = 0.0001 and 0.043 in D and ND ART, respectively), duration on dialysis (P = 0.0001 across board in D and ND ART as well as in D and ND VRT, respectively), and plasma urea (PU) and plasma creatinine (PCr) (P < 0.05). Factors found to be associated with memory impairment included age (P = 0.045 and 0.025 on words and figures RMT, respectively), PU (P = 0.002 and 0.005 on words and figures RMT, respectively), and PCr (P = 0.012 and 0.040 on words and figures RMT, respectively). Duration on dialysis (P = 0.032) and PCr (P = 0.001) were associated with attention and concentration. Only psychomotor speed was independently predicted by duration of CKD.
Conclusion:
Factors associated with psychomotor speed impairment were duration of CKD, duration on dialysis, and PU and PCr while age, PU, and PCr were associated with memory. Duration on dialysis and PCr were associated with attention and concentration. Only psychomotor speed was independently predicted by duration of CKD.
Keywords
Associated factors
chronic kidney disease
Nigeria
predictors
INTRODUCTION
Chronic kidney disease (CKD) is a substantial public health problem.[1] Epidemiologic data increasingly show that individuals at all stages of CKD have a higher risk of developing cognitive dysfunctions.[2] In patients on maintenance hemodialysis, the prevalence of cognitive dysfunctions ranges between 30% and 60% and twice that of the age-matched control.[34] Cognitive impairment (CI) is important because it is also likely to become more of a problem as the dialysis population ages.[5]
Deficits in a number of domains of cognition including concentration, judgment, abstraction, memory, and executive function may occur in patients with CKD and have implication for patients’ capability ranging from impaired ability to participate in adequate care of their condition,[4] to difficulty with requisite provision of informed consent in relation to dialysis initiation or maintenance, and ultimately, renal transplantation.[36] In spite of the huge influence of CI on the quality of life of patients with CKD, it is poorly recognized by nephrologists.[7]
Prior studies have reported an association of CKD with cognitive performance, incident dementia, and functioning cognitive capabilities in relation[7] to verbal learning, visual attention, mental flexibility, and executive functioning.[389] In some of these studies, CI assessment was based on short screening tests, such as the mini-mental state examination,[1011] which is adjudged to have limited sensitivity for cognitive function evaluation.[912] Besides, some of the cognitive tests were administered during a hemodialysis session. However, Murray et al. have shown that global cognitive function varies significantly over the course of the dialysis session with the performance worst during the session itself and best shortly before the session or on the day after.[913]
Moreover, there is small number of published studies of predictors of cognitive dysfunctions patients with CKD from the developing countries, where CKD constitutes a major public health problem. Consequently, there is a need for information about CI and its associated factors among CKD patients which will draw attention to early evaluation, diagnosis, follow-up, and treatment.
Against this background, this study was designed to determine the factors associated with CI and factors that independently predict CI in CKD patients on maintenance hemodialysis using a computer-based neuropsychological test battery.
MATERIALS AND METHODS
Participants and study design
The study was a two-stage cross-sectional in design. The first stage included recruitment of 100 apparently healthy participants aimed at determining the reference values for the different test battery used in the study. The second stage of the study included eighty CKD patients on maintenance hemodialysis who were recruited consecutively from the dialysis unit of Aminu Kano Teaching Hospital (AKTH) during the study period (from June 2010 to June 2014).
The exclusion criteria were age <18 years, less than primary school education, presence of comorbidities that could cause cognitive dysfunction (e.g., epilepsy, cerebrovascular disease, Parkinson's disease, epilepsy, human immunodeficiency virus, brain tumor). Cases with psychiatric disorders were also excluded. Other exclusion criteria included drug abuse, current use of psychoactive drugs, history of previous head injury with loss of consciousness, severe functional impairment (Karnofsky performance <50% alcohol intake, and presence of cardiac failure), and use of anticholinergic medications.
Data sources
Using a questionnaire-based pro forma, we obtained information on sociodemographic characteristics and CKD-related information, history of hypertension, heart disease, previous stroke, and diabetes. Subsequently, all the patients had a detailed general physical and neurological examination and later screening for functional performance and depression using Karnofsky scale and Hamilton and anxiety depression scale (HADS), respectively. Those with Karnofsky performance more than 50% and no evidence of depression on HADS proceeded with the study. Results of blood count, coagulation tests, serum electrolytes, glucose, serum urea, and creatinine levels were also obtained in the patients.
Cognitive function assessment
The cognitive assessment was conducted with the aid of a computer-assisted neuropsychological test battery called Iron Psychology (“FePsy”).[14] FePsy had been validated for use in studies of cognitive functions.[1516] The FePsy test has been validated for use among Nigerians and in patients with chronic renal impairment.[816] The tests employed in the present study included simple reaction time task, binary choice reaction time, computerized visual scanning task, recognition memory task, and finger-tapping test. The tests were administered in a quiet and well-lit room at a room temperature with the case sitting at distance of 40–60 cm from the visual display screen of the computer. The memory function was assessed using the recognition memory test (RMT-word and figure). Mental or psychomotor speed was assessed using the simple reaction time test (auditory reaction time [ART] and visual reaction time [VRT]). Binary choice task (BCT), which is a complex form of the continuous performance test, was used in the study to assess psychomotor speed, attention, and concentration. The computer-assisted visual scanning task (CVST), which could be used to detect the presence of brain damage in the case, reflects accuracy and speed of responses, and is evaluated within the context of visual (complex) information processing and perceptual-mental strategies.[16] Finger-tapping test was also performed in the context of motor activation and fluency. All cognitive assessments were conducted shortly before dialysis sessions.
The interpretation of the results of the cognitive assessment is such that the higher the mean scores on auditory reaction test, visual reaction test, and computerized visual search task, the poorer the cognitive performance. Conversely, the higher the mean scores on recognition test, binary choice test, and tapping task (which are expressed as correct percent of total), the better the cognitive performance. On conducting these neuropsychological tests, care was taken to ensure good brightness and contrast of screen, sufficient sound level of the speaker and to elucidate a history of photosensitive seizure from the patients.
Data analysis
Analysis of data was done using GraphPad Prism version 5.03 (GraphPad Software, Inc. CA 92037 USA). The normality of the numerical data was assessed using D’Agostino and Pearson Omnibus tests. Numerical data that were normally distributed were expressed as the mean ± standard deviation (SD). Reference values of the neuropsychological test battery were obtained from the first phase of the study. The reference range of the neuropsychological test battery was set by the 2½ and 97½ percentiles so that reference ranges contain the central 95% of the distribution. Comparisons of cognitive function parameters between CKD patients with normal and abnormal cognitive performance were performed using Student's independent sample t-test and Mann–Whitney test in the case of parametric and nonparametric data, respectively. P < 0.05 was considered statistically significant. The variables that were significant on univariate analysis were subjected to multiple logistic regression model and the covariates were adjusted for each independent (regression) variable to find independent predictors of CI.
Informed consent was obtained from every participant and ethical approval was obtained from the Ethical Review Committee of the AKTH, Kano.
RESULTS
During the study period, eighty patients comprising 45 (56.2%) males and 35 (43.8%) females were evaluated. The mean age ± SD of the patients was 49.85 ± 10.58. The median duration of CKD from diagnosis was 2 years (range 0.2–5 years) [Table 1].
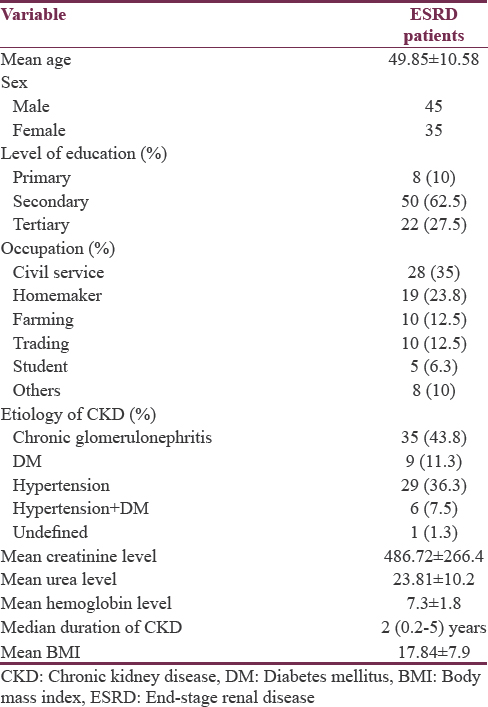
Considering the etiological factors of CKD in the patients, 35 (43.8%) had chronic glomerulonephritis, 29 (36.2%) patients had hypertension only, 9 (11.2%) had type 2 diabetes mellitus only, 6 (7.6%) had hypertension and diabetes, and 1 (1.2%) patient had unidentified etiology.
The reference values obtained from 100 apparently healthy volunteers for ART D, ART ND, VRT D, VRT ND, BCT, CVST, RMT (words), RMT (figure), taping test D, taping test ND were 149.7–338.8, 158.6–290.4, 86.4–275.5, 86.4–275.5 ms, 49.8%–92.2%, 4.0–10.4 ms, 39.62%–97.11%, 17.62%–88.42%, 39.8%–82.19%, 36.39–78.77, respectively. Table 1 shows sociodemographic, clinical, and laboratory characteristics of the patients.
Forty-one (51.3%) and 43 (53.8%) CKD patients had abnormal scores on ART dominant (D) and nondominant (ND) sides, respectively. Forty (50%) and 42 (52.5%) patients had abnormal scores on VRT D and ND sides, respectively. Twenty-one (26.3%) and 68 (85%) had abnormal scores on BCT and CVST, respectively. Sixty-four (80%) and 65 (81.3%) had abnormal scores on RMT (words) and RMT (figure), respectively. Fifty-two (65%) and 48 (60%) patients had abnormal scores on D and ND sides of tapping test, respectively.
Factors associated with psychomotor speed impairment were duration of CKD from diagnosis (P = 0.0001 and 0.043 in D and ND ART testing, respectively), duration of dialysis use (P = 0.0001 across board in D and ND ART testing as well as in D and ND VRT testing, respectively), and plasma urea (PU) and plasma creatinine (PCr) levels (P < 0.05).
Factors found to be associated with short-term memory impairment included the age (P = 0.045 and 0.025 on words and figures RMT testing, respectively), PU (P = 0.002 and 0.005 on words and figures RMT testing, respectively), and serum creatinine (P = 0.012 and 0.040 on words and figures RMT testing, respectively). Duration of dialysis use (P = 0.032) and serum creatinine (P = 0.001) were associated with attention and concentration in the patients. Tables 2 and 3 show the detail of factors associated with performance on the various neuropsychological tests. Following adjustment for confounders, out of the entire cognitive domains assessed, only psychomotor speed was independently predicted by duration of CKD [Figure 1].

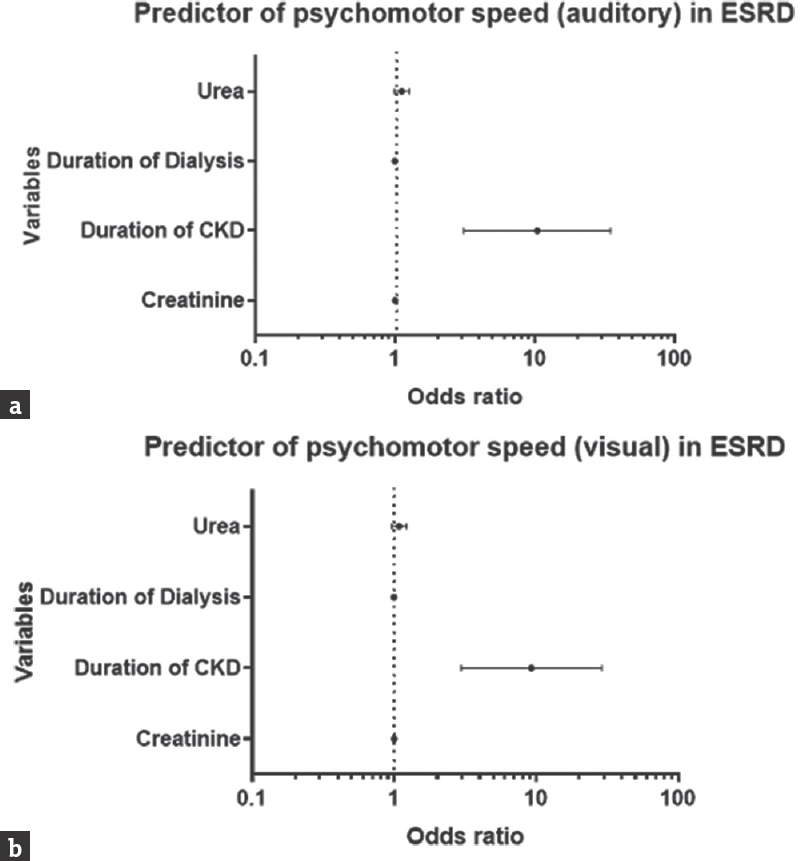
- Odd ratio plot showing an independent predictor of psychomotor speed: Auditory (a), visual (b)
DISCUSSION
On the basis of this cross-sectional observation conducted in CKD patient on maintenance hemodialysis, we showed that majority of the CKD patients had performance on neuropsychological test batteries that were below the normative values generated from the same population. The high frequency of CI is not dissimilar to the previous reports of 30%–70% among hemodialysis patients.[1718] This study extends the findings of previous cross-sectional and longitudinal studies that reported a close association between CKD and CI.[31920] In a prospective cohort study conducted in a multiethnic stroke-free population by Khatri et al., CKD was found to be associated with cognitive decline and the relationship extended to those with mildly reduced estimated glomerular filtration rate.[17] Several mechanisms underlying CI in persons with CKD have been hypothesized; one of them proposed that mediators of relations between kidney function and cognitive functions are similar to those that have been advanced to explain relations between cardiovascular risk factors and cognition,[8] and this has paved the way for investigations of CKD in relation to diseases where cardiovascular risk factors may play a causal role.[17] A plausible potential mechanism is that CKD, through its adverse effects on the cerebral vasculature, potentiates vascular CI.[212223] A systematic review of structural and functional neuroimaging findings in patients with CKD concluded that cerebral atrophy and cerebral density change, cerebral vascular disease, including deep white matter hyperintensities, silent cerebral infarction and cortical infarction, white matter lesions, and cerebral microbleeds,[24] which are known to be associated with depression and CI,[25] are frequently seen in patients with CKD.
Our data showed that longer CKD duration is associated with an increased likelihood of poor performance on auditory and visual reaction testing, the tests that assessed psychomotor speed in the participants. This observation was supported by both univariate analysis and multiple logistic regression. However, we did not demonstrate a similar effect on attention, memory, motor activation and fluency, and processing and perceptual-mental strategies. Our current finding is concordant with the work of the previous workers.[56726]
Besides cerebrovascular changes, a direct uremic neuronal toxicity supports additional neurodegenerative pathways.[2] In the current study, urea, creatinine, and duration on dialysis were also found to be significantly associated with cognitive dysfunction in the patients but were not strong enough to predict impairment in the study. Unlike in the general population and contrary to expectation, age was not established as a strong factor and determinant of impaired cognitive function in patients with CKD on maintenance dialysis. The significant relationship that was found to exist between age and performance on auditory reaction testing was not sustained on adjusting for other associated factors. This finding is in conformity with the reports from studies elsewhere.[56726]
A major constraint in CI in CKD studies is the definition of normal cognitive performance in the study population; the current study obtained the baseline normative values from age, sex, and level of education-matched apparently healthy individuals in the study population, which were subsequently used in categorization of the cognitive performance in the selected sample of patients. This, in our view, is one of the strengths of the current study.
Nonetheless, the current study has some limitations; the presence of some other coexisting conditions that our analysis did not reveal but which may have impacted cognition could not be completely excluded. The cross-sectional study design of this study limits ability to make inferences regarding the cause of the poor performance observed in some of the participants. It would have been more instructive to track this relationship over time; hence, the need for a longitudinal study on the subject matter cannot be overemphasized.
In spite of these limitations, the findings in our study help raise awareness in respect to the reality of the high frequency of CI, factors associated with impaired cognitive domains, and determinants of CI in CKD patients. It is high time the physicians in charge of CKD patients respond by ensuring early screening for CI, using simple instruments, and ultimately reflect the screening results in the management of patients as well as in discussions with their caregivers.
CONCLUSION
We showed that the factors associated with psychomotor speed impairment were duration of CKD, duration on dialysis use, and PU and PCr levels. Factors found to be associated with short-term memory impairment included the age, PU, and serum creatinine levels. Duration on dialysis and serum creatinine were associated with attention and concentration in the patients, and only psychomotor speed was independently predicted by duration of CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353-63.
- [Google Scholar]
- Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41-9.
- [Google Scholar]
- Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol. 2006;1:1000-5.
- [Google Scholar]
- Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011;79:14-22.
- [Google Scholar]
- Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446-52.
- [Google Scholar]
- Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216-23.
- [Google Scholar]
- Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98.
- [Google Scholar]
- Is the Montreal cognitive assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-6.
- [Google Scholar]
- Correlates and outcomes of dementia among dialysis patients: The dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2006;21:2543-8.
- [Google Scholar]
- Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis. 2008;15:123-32.
- [Google Scholar]
- A neuropsychological test battery for the Apple IIE. Int J Man Mach Stud. 1986;25:453-86.
- [Google Scholar]
- Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology. 1991;41:1608-16.
- [Google Scholar]
- Predictive Validity and Usefulness of Visual Scanning Task in HIV/Aids – A Case Control Analysis. 2008. Afr J Neurol Sci. 26:45-52. Available from: http://www.ajol.info/indexphp/ajns/article/view/7593
- [Google Scholar]
- Cognitive impairment in CKD: No longer an occult burden. Am J Kidney Dis. 2010;56:615-8.
- [Google Scholar]
- Kidney disease as a determinant of cognitive decline and dementia. 2015. Alzheimers Res Ther. 7:29. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4360943/
- [Google Scholar]
- Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73:920-7.
- [Google Scholar]
- Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215-22.
- [Google Scholar]
- Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55-61.
- [Google Scholar]
- Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol. 2013;8:1429-48.
- [Google Scholar]
- Depressive symptoms in hemodialysis patients with silent cerebral infarction. Psychosomatics. 2003;44:352-3.
- [Google Scholar]
- Duration of CKD and executive function in pediatric patients. 2015. Kidney Int. 87:800-6. Available from: http://www.medscape.com/viewarticle/843713
- [Google Scholar]






