Translate this page into:
Prognostic Factors in Elderly Patients with High-grade Gliomas: A Retrospective Analysis of 24 Cases
Address for correspondence: Dr. Meenu Gupta, Department of Radiotherapy, Cancer Research Institute, Swami Rama Himalayan University, Doiwala, Dehradun - 248 016, Uttarakhand, India. E-mail: meenugupta.786@rediffmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
Due to the aging of the population, diagnosis of high-grade gliomas (HGGs) in the elderly is becoming more common. The purpose of this study was to report our experience in 24 elderly patients with HGGs and evaluate the value of different prognostic factors.
Design and Setting:
Retrospective analysis of 24 elderly patients of ≥60 years with newly diagnosed HGGs, who were treated at our department between January 2009 and December 2012, was done.
Patients and Methods:
Age, gender, Karnofsky performance scale (KPS) score, extent of surgery, and use of temozolomide were evaluated using univariate and multivariate analyses. Survival was determined using the Kaplan–Meier method, and differences were compared using the log-rank test. Cox regression analysis was conducted to identify the independent prognostic factors.
Results:
The median overall survival of the patient cohort was 10 months. The 1- and 2-year survival rates were 45.8% and 16.6%, respectively. The analysis revealed that KPS score and use of concomitant chemotherapy were significant prognostic factors.
Conclusion:
The results of our analyses demonstrate that KPS score and use of concomitant chemotherapy yield encouraging outcomes in elderly patients with HGGs, validating the results published in research papers.
Keywords
Elderly
high-grade glioma
prognostic factors
INTRODUCTION
The progressive aging of the population is resulting in a continuous increase in the incidence of gliomas in elderly people, especially the most frequent subtype, glioblastoma multiforme (GBM).[1] Data from retrospective studies and meta-analysis suggest that elderly patients with high-grade gliomas (HGGs) have a poorer outcome than younger patients, possibly because of the presence of comorbidities, resistance to cancer therapy, genetic aberrations, neurodegeneration, or age discrimination. Older patients constitute approximately one-half of the patients with malignant gliomas. The prevalence of GBM increases with age. Among individuals aged between 65–74 years and 75–84 years, the incidence is 13.27 and 14.49 per 100,000 person-years, respectively.[2] Between 1983 and 1990, there was a 5% per year increase in the incidence of malignant astrocytomas among patients older than 65 years in a review of six French cancer registries.[3] A study of the surveillance, epidemiology, and end results registry database of patients diagnosed with cancer between 1973 and 2000 showed an increase in the incidence of GBM, with the fastest increase occurring among elderly patients (≥70 years).[4] Despite aggressive treatment, the median survival among all GBM patients is only 12–15 months from diagnosis. Among elderly patients, median survival is markedly reduced at only 4–5 months, according to population-based studies. Growing research demonstrates that GBM among elderly patients has less favorable molecular signatures, as compared with younger patients.[5]
Various patient and tumor characteristics such as patient's age, Karnofsky performance status (KPS), tumor location, size, and presence of seizures are significant prognostic indicators in patients with malignant gliomas.[6] The extent of surgical resection, the volume of the postoperative residual tumor, dose of radiation therapy, and chemotherapy are other significant prognostic factors. Currently, there is no accepted standard treatment of GBM in elderly patients.[7] The optimal fractionation regimen has not been defined yet. Therapeutic approaches are directly influenced by the prognostic factors (age, grade, KPS, and comorbidity).[8] Worse prognosis in elderly glioblastoma patients may be linked to different patterns of care.[9] The objective of the current study was to evaluate the value of different prognostic factors and patterns of care in patients aged ≥60 years with HGGs.
PATIENTS AND METHODS
We conducted a retrospective analysis of 24 patients with age ≥60 years with newly diagnosed HGGs who were treated at our Department between January 2009 and December 2012. The study protocol was approved by the research committee of our university.
Data collection
The following data were collected from the medical records of patients (1) demographic profile (age and gender); (2) presenting symptoms and duration; (3) site of tumor; (4) type of surgery; (5) KPS before radiation; (6) type of postoperative treatment (radiotherapy [RT] +/-chemotherapy); (7) follow-up data: clinical outcomes including overall survival, which was mainly collected when patients visited the outpatient department/hospital information system or during phone interview with patients and/or relatives. The following prognostic factors were analyzed age, sex, performance status, extent of surgery, and use of concomitant temozolomide.
Treatment details
Each patient was immobilized in a customized thermoplastic head mask and treatment planning contrast-enhanced computed tomography (CT) images was performed. The image data set was transferred to three-dimensional (3D) planning system. The gross target volume (GTV) was defined by contrast-enhanced lesion on preoperative CT/magnetic resonance imaging (MRI). The clinical target volume (CTV) consisted of GTV + 2–3 cm safety margin. The planning target volume was CTV plus a margin of 0.5–1 cm. We planned the minimum and maximum absorbed dose to be between 95% and 105%, and a dose-volume histogram was generated.
Focal RT was delivered on 6-MV Linear accelerator on the basis of 3D treatment planning or intensity-modulated RT. Conformal RT was given at 2 Gy/day, 5 days a week. Median external beam RT dose given was 60 Gy/30 fractions. Only two patients received 30 Gy/10 fractions. Ten patients received concurrent temozolomide 100 mg daily. During RT, all patients received 12–24 mg dexamethasone and 300 mg diphenylhydantoin. Only eight patients received adjuvant temozolomide 150 mg/m2 on days 1–5 with a 28 days interval. The treatment was continued for six cycles. During RT, all the patients were assessed on a weekly basis by the radiation oncologist.
Statistical analysis
Statistical analysis was done using SPSS 22, IBN Corp, Armonk, NY, USA. Survival was measured from the time of initial operation until the patient died or until the final analysis. Survival rates were determined by Kaplan–Meier method. Differences between survival curves were analyzed by the log-rank test. Uni- and multivariate analysis was conducted using the Cox proportional hazard model. P < 0.05 was considered to indicate statistical significance.
RESULTS
The patient distribution regarding the examined factors and the patient characteristics are presented in Table 1. The mean age was 65 years (range 60–80 years, median 63.5 years) and the male: female ratio was 1.18:1. Twenty-One (87.5%) patients were histologically confirmed as GBM, two (8.3%) patients were confirmed as anaplastic astrocytomas, and only one (4.1%) patient was confirmed as oligoastrocytoma grade III. The majority of patients 14 (58.33%) had KPS 70 or more. The median KPS before RT was 70 (range 40–90). All patients underwent surgery. Gross total surgical resection was done in 10 (41.66%) patients, while partial resection or biopsy was performed in 14 (58.33%).
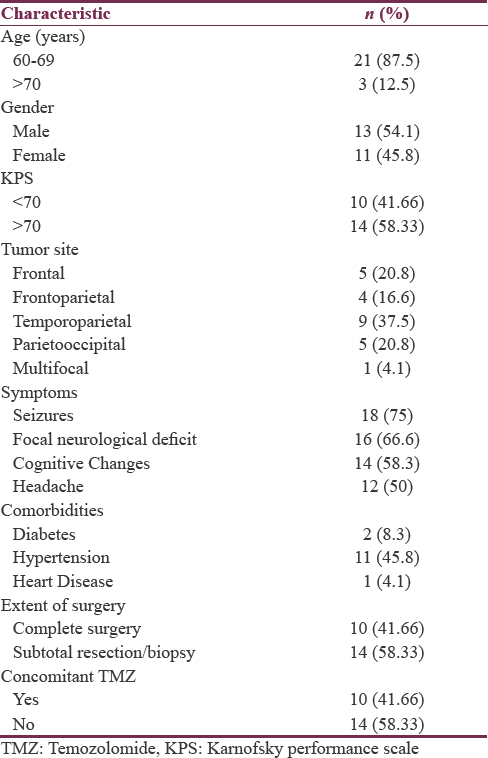
The majority of patients presented by a combination of symptoms including seizures in 18 (75%), focal neurological deficits in 16 (66.6%), cognitive changes in 14 (58.3%), and headache in 12 (50%) patients. The median actuarial survival for the entire study population was 10 months. The overall survival at 3 months, 6 months, 1 year, and 2 years was 91.6%, 66.6%, 45.8%, and 16.6%. Four patients were alive at the time of final analysis. The results of the survival analysis are summarized in Table 2. The analysis showed that performance status and administration of chemotherapy were significant prognostic factors. Multivariate analysis using Cox regression model is summarized in Table 3 along with the respective hazard ratios and confidence intervals.
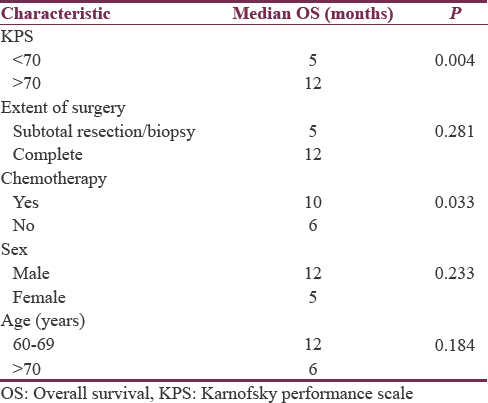
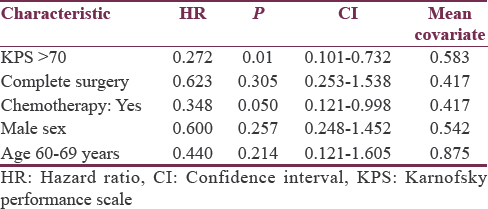
With per decade increase in age median survival decreases from 12 months for 60–69 years to 6 months for the age of 70–80 years with P = 0.184. The median overall survival was better in male patients 12 months versus 5 months in female patients with P = 0.233. The median survival for patients with a KPS score of ≥70 was 12 months while that for patients with a KPS score of <70 was only 5 months. A statistically significant difference was observed in the median survival according to the KPS score (P = 0.004) [Figure 1]. The type of surgery revealed a difference in median survival between biopsy/subtotal resection and complete resection (5 and 12 months, respectively, P = 0.281). The addition of concurrent Temozolomide to radiation treatment in these patients found a difference in duration of the median overall survival (median survival 10 months with concomitant chemo RT vs. 6 months with radiation as sole modality, P = 0.033) [Figure 2].
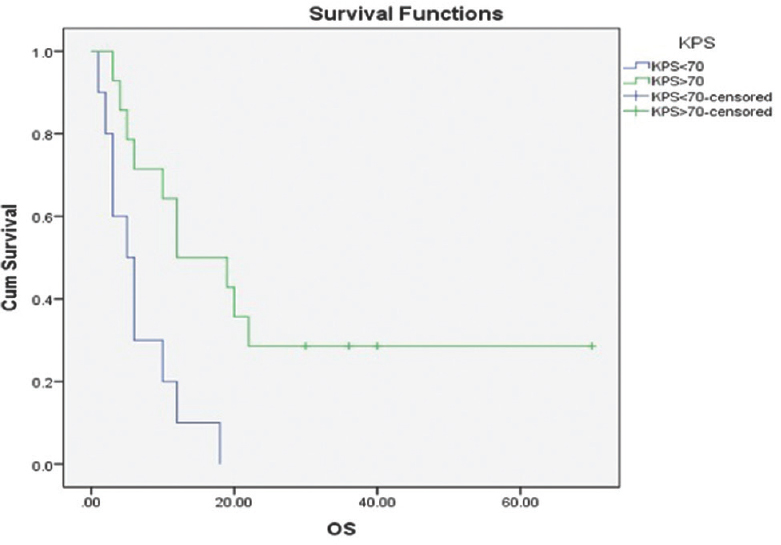
- Kaplan–Meier estimates of overall survival KPS<70 versus KPS>70. P = 0.004 (log-rank test)
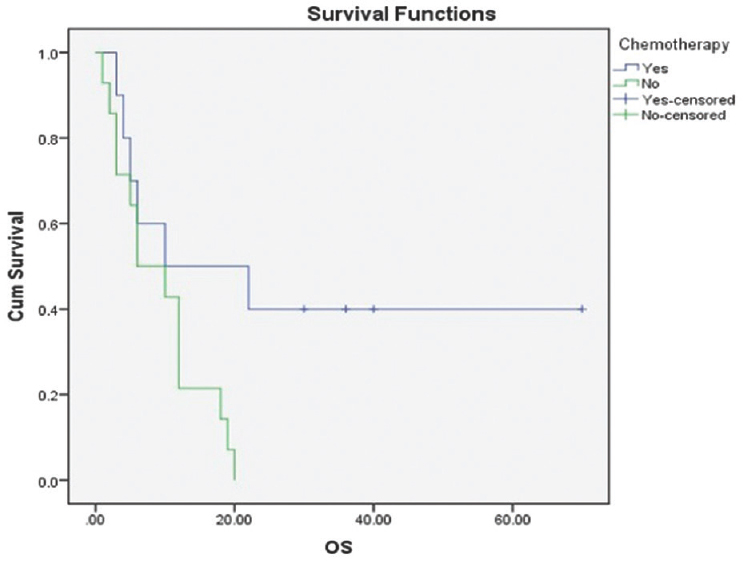
- Kaplan–Meier estimates of overall survival concomitant versus no chemotherapy P = 0.033 (log-rank test)
DISCUSSION
HGGs are an evenly fatal tumor associated with a poor prognosis. However, patients aged 65 years or older represent half of all patients with this illness, and this percentage is going to grow in the next few decades, due to the aging of the general population. Some reviews demonstrated that age is the most significant predictor for resection, RT (RT) or chemotherapy and that advancing age is associated with decreasing use of all three modalities and increasing of best supportive care only.[9] The 6 month, 1 year, and 2-year overall survival obtained for our elderly patients was 66.6%, 45.8%, and 16.6%, respectively which is in line with published series. Mohan et al.[10] reported a median survival of 7.3 months in 58 patients who were 70-year-old treated with standard RT. Villà et al.[11] reported an overall survival of 8 months in 18 elderly patients of 70 years treated by standard RT. Retrospective studies strongly suggest that patients with subtotal resection do not live longer than as those with gross total resection. In a large retrospective study of 416 patients with GBM, who were treated at M. D Anderson hospital, a volumetric analysis of the extent of resection on postoperative MRI showed at least 98% tumor resection resulted in a survival advantage compared with less complete resection (13 months vs. 8.8 months).[12] A study conducted in France by Keime-Guibert et al. enrolled a total of 81 patients 70 years of age or older with good functional status. Forty-two received comfort care alone; the other 39 patients received supportive care and radiation therapy (50 Gy in doses of 1.8 Gy/day, given 5 days a week). Patients receiving radiation therapy had a median survival of 29.1 weeks compared with 16.9 weeks for those receiving supportive care alone. Radiation therapy produced a survival benefit regardless of the extent of surgery performed, which ranged from biopsy alone to complete resection.[13] In the present study, extent of surgery was found to be a prognostic factor with median survival 12 months for patients who underwent complete resection versus 5 months for patients with subtotal resection/biopsy. Patients with HGG can be classified as having a favorable prognosis (younger or with good performance status) or a poor prognosis (older or with poor performance status) with median survival of 12–24 and 6–9 months, respectively. The standard management for the favorable subgroup is maximum safe resection followed by adjuvant conventionally fractionated RT, with or without chemotherapy.[14]
In 2010 ASCO, Malmström et al. presented their data and later published which included the newly diagnosed GBM patients age ≥60 years with performance status of 0–2, randomized to either standard radiation (60 Gy in 2 Gy fractions over 6 weeks) or hypofractionated radiation (34 Gy in 3.4 Gy fractions over 2 weeks) or 6 cycles of chemotherapy with TMZ (200 mg/m2 day 1–5 every 28 days). There was no significant difference in overall survival between the three treatment arms, with median survival being 8 months for TMZ, 7.5 months for hypofractionated RT, and 6 months for 6 weeks RT (P = 0.14). They concluded that elderly patients with GBM have a short survival. Time-consuming therapy that does not offer longer survival should, therefore, be avoided.[15] These results indicate that standard RT should no longer be offered to the elderly patient population with GBM. RT was well tolerated at full dose in the majority of our patients and addition of temozolomide could give benefit in overall survival, but as this was a retrospective study and at that time the trend toward using hypofractionated RT in elderly patients was low. For older patients who are not candidates for a combined modality approach because of poor functional status or significant comorbidity, literature suggests shorter courses of radiation therapy, which will be more convenient and may offer an advantage because of decreased toxicity. Recently, the results of the elderly Glioblastoma Trial (Canadian Cancer Trials Group ce. 6 study, European Organization for Research and Treatment of Cancer 26062-22061 study, and Trans Tasman Radiation Oncology Group 08.02 study) presented at the 2016 American Society of Clinical Oncology annual general meeting, provided further insight into the role of combined chemo RT in older patients with GBM. However, many questions remain unresolved, including the optimal fractionation schedule for RT, the role of temozolomide as monotherapy, and the most appropriate definition of “elderly” for clinical decision-making in this setting.[16]
Seizures in elderly patients can lead to serious consequences; all efforts should be made to keep these patients seizure free. In this study, all patients received phenytoin 300 mg daily. The newer drugs, such as levetiracetam, gabapentin, and tiagabine, are more suitable choices for elderly patients.[17] In agreement with other studies, we also found that KPS and addition of chemotherapy are the most important prognostic factors. Maximal safe resection followed by radical RT and temozolomide might be the optimal treatment of choice since glioblastoma-diagnosed patients over 60 years of age showed a statistically significant survival benefit which was concurred by data published by Beramendi et al.[18]
Although complete surgical resection also showed a trend toward better survival, this required more sample size. Advanced age is accepted as one of the most pejorative prognostic factors in patients with HGGs. In fact, there is some evidence suggesting that tumorigenic pathways to GBM vary with the age of the patient.[19] The study has clinical limitation in the fact that it is retrospective, it has a small sample, and it lacks the evaluation of the gene expression signature of the tumor.
CONCLUSION
The study demonstrated a benefit of adding radiation therapy and adjuvant Temozolomide for elderly patients with HGGs. The numbers were too small to clearly demonstrate the optimal regimen. Higher KPS and surgical resection increased survival. Overall prognostic considerations should be taken into account to guide treatment recommendations for individual patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004;101:2293-9.
- [Google Scholar]
- Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging. 2014;9:357-67.
- [Google Scholar]
- Conformal hypofractionated radiotherapy concomitant with and followed by temozolomide in high grade glioma in elderly patients. Med J Cairo Univ. 2012;80:799-805.
- [Google Scholar]
- Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst. 2013;25:21-30.
- [Google Scholar]
- Comorbidity assessment and adjuvant radiochemotherapy in elderly affected by glioblastoma. Med Oncol. 2012;29:3467-71.
- [Google Scholar]
- Outcome in elderly patients undergoing definitive surgery and radiation therapy for supratentorial glioblastoma multiforme at a tertiary care institution. Int J Radiat Oncol Biol Phys. 1998;42:981-7.
- [Google Scholar]
- Efficacy of radiotherapy for malignant gliomas in elderly patients. Int J Radiat Oncol Biol Phys. 1998;42:977-80.
- [Google Scholar]
- A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
- [Google Scholar]
- Poor-prognosis high-grade gliomas: Evolving an evidence-based standard of care. Lancet Oncol. 2002;3:557-64.
- [Google Scholar]
- Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916-26.
- [Google Scholar]
- A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma. J Clin Oncol. 2016;34(18 suppl)
- [Google Scholar]
- Choice of antiepileptic drugs for the elderly: Possible drug interactions and adverse effects. Expert Opin Drug Metab Toxicol. 2012;8:81-91.
- [Google Scholar]
- Prognostic factors and survival study in high-grade glioma in the elderly. Br J Neurosurg. 2016;30:330-6.
- [Google Scholar]
- Malignant astrocytomas of elderly patients lack favorable molecular markers: An analysis of the NOA-08 study collective. Neuro Oncol. 2013;15:1017-26.
- [Google Scholar]






