Translate this page into:
Prevalence of Cerebral Microbleeds in Thai Patients with Ischemic Stroke
Address for correspondence: Prof. Pornpatr A. Dharmasaroja, Department of Internal Medicine, Division of Neurology, Faculty of Medicine, Thammasat University, Thailand. E-mail: pornpatr1@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
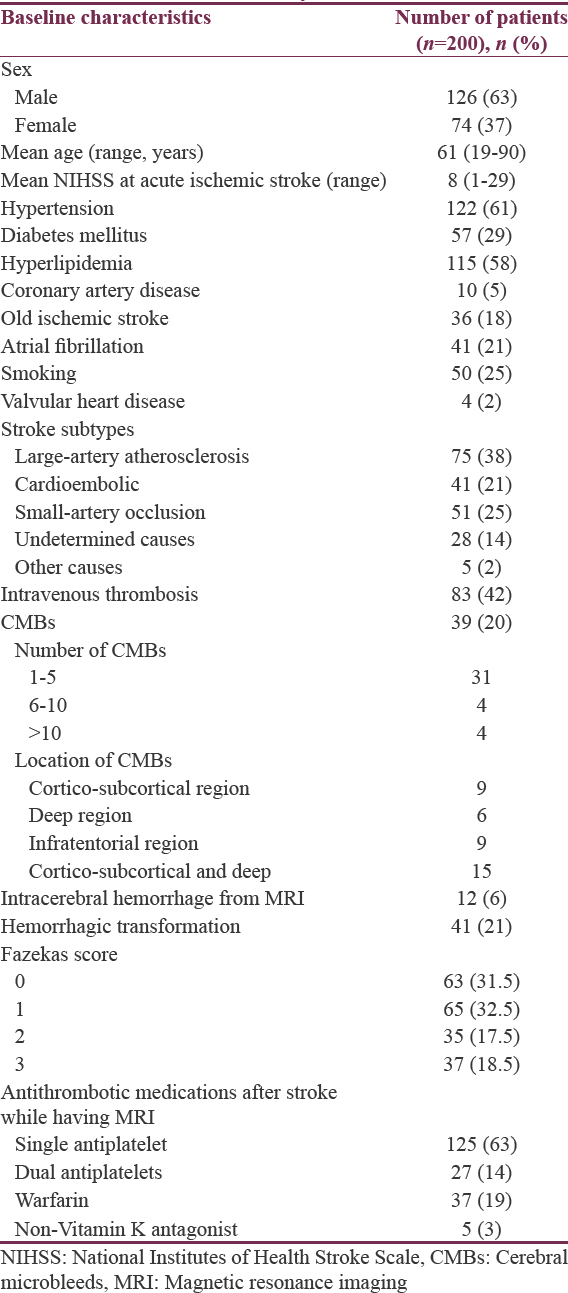
With the widespread use of magnetic resonance imaging (MRI), cerebral microbleeds (CMBs) are commonly detected. Ethnicity seems to play a role in the prevalence of CMB, with higher prevalence in participants from Asian origin. The purpose of the study is to look for the prevalence of CMBs and associated factors in Thai patients with ischemic stroke.
Methods:
Patients with acute ischemic stroke who had MRI and magnetic resonance angiography during January–August 2014 were included in the study. T2*-weighted gradient-recalled echo was used to define CMBs. Baseline characteristics, stroke subtypes, and severity of white matter lesions were compared between patients with and without CMBs.
Results:
Two hundred patients were included in the study. Mean age of the patients was 61-year-old. Mean National Institutes of Health Stroke Scale was 8. The prevalence of CMBs was 20% (39/200 patients). Hypertension (odds ratio [OR] 3.05, 95% confidence interval [CI] 1.07–8.68, P = 0.037), and moderate-to-severe white matter lesions (Fazekas 2–3, OR 7.61, 95% CI 3.06–18.95, P < 0.001) were related to the presence of CMBs.
Conclusions:
CMBs were found in 20% of patients with ischemic stroke, which was lower than those reported from Japanese studies but comparable to a Chinese study. CMBs were associated with hypertension and severity of the white matter lesions.
Keywords
Asian
cerebral microbleed
ischemic stroke
Thai
INTRODUCTION
Cerebral microbleeds (CMBs) are defined as small perivascular hemosiderin deposits.[1] With the widespread use of magnetic resonance imaging (MRI), CMBs are more commonly detected. In studies using sensitive MRI technique, the prevalence of CMBs is as high as 11.1%–23.5% in community-dwelling elderly people.[23] Prevalence is higher in patients with ischemic stroke (40%) and spontaneous intracerebral hemorrhage (ICH) (47%–80%). Ethnicity seems to play a role in the prevalence of CMB, with higher prevalence in participants from Asian origin.[4] CMBs are related to age, hypertension, leukoaraiosis, amyloid angiopathy, atrial fibrillation, and some genes.[14]
Findings of CMBs, especially in stroke patients, may be important. Some stroke patients will have a recurrent stroke, and intravenous thrombolysis is the standard treatment in eligible patients within 4.5 h of stroke onset. Intravenous thrombolysis increases symptomatic and asymptomatic ICH, even in patients without baseline CMBs. A recent meta-analysis showed that the presence of CMBs on pretreatment MRI scans in patients with acute ischemic stroke increased risk of symptomatic ICH after intravenous thrombolysis (odds ratio [OR] 2.87, 95% confidence interval [CI] 1.76–4.69, P < 0.0001).[5] However, the authors concluded that although the presence of CMBs increased the risk of ICH after thrombolytic treatment, detecting CMBs should not prevent thrombolytic treatment based on present evidence.
There has been no study about CMBs in Thai patients with ischemic stroke. The purpose of this study is to look for the prevalence of CMBs and associated factors in Thai patients with ischemic stroke.
METHODS
Patients who presented with acute ischemic stroke and had MRI and magnetic resonance angiography (MRA) during January–August 2014 at Thammasat University Hospital were included in the study. MRI was performed using a 1.5 Tesla Philips Achieva XR Scanner with a neurovascular 16-channel radiofrequency head coil. A T2*-weighted gradient-recalled echo (GRE) sequence (TR 700 ms, TE 23 ms, and flip angle of 18°) with a 5-mm slice thickness and 1 mm gap was used to define hemorrhage. GRE images were acquired from axial and coronal orientation. Small (<10 mm), hypointense, well-demarcated, rounded lesions on GRE were used to define CMBs. If the hypointense lesions on GRE were larger than 10 mm, these were defined as ICH. If the hypointense lesions on GRE were within the infarct lesions, these were defined as hemorrhagic transformation [Figure 1]. Locations of CMB, ICH, and hemorrhagic transformation were recorded. Fazekas scale (0–3) was used to classify severity of white matter lesions; with higher score, more confluent of the lesions.[67] Fazekas 0: None or a single punctate white matter hyperintensity lesion, Fazekas 1: Multiple punctate lesions, Fazekas 2: Beginning confluency of lesions (bridging), and Fazekas 3: Large confluent lesions [Figure 2]. Fazekas was scored on transverse T2/fluid attenuation inversion recovery images (TR 11,000 ms, TE 140 ms).
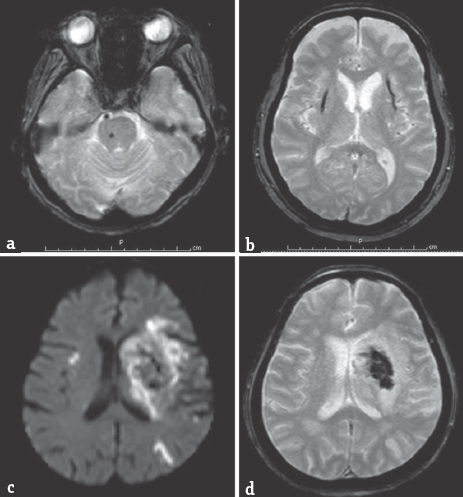
- (a) Cerebral microbleed at rightside of pons from gradient-recalled echo; (b) Intracerebral hemorrhage at bilateral putaminal hemorrhages from gradient-recalled echo; (c) (diffusion-weighted imaging); (d) (gradient-recalled echo) acute hemorrhagic infarct at left middle cerebral artery territory
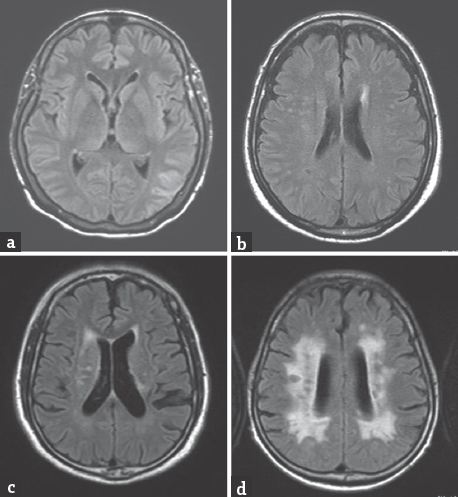
- (T2/fluid attenuation inversion recovery images); (a) Fazekas scale 0; (b) Fazekas 1; (c). Fazekas 2; (d) Fazekas 3
All patients with acute ischemic stroke were treated per standard care protocol. Intravenous thrombolytic treatment was given in eligible patients. Either antiplatelet or anticoagulant was prescribed in most patients who had no contraindication. Stroke subtypes were classified by TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria: Large-artery atherosclerosis (LAA), cardioembolism (CE), small-artery occlusion (SAO), stroke of other determined cause, and stroke of undetermined cause. Severity of stroke was evaluated by the National Institutes of Health Stroke Scale (NIHSS). Baseline characteristics of patients, stroke subtypes, antithrombotic medications, and all imaging data were collected and studied.
The data were presented as a mean for the continuous variables and a percentage (number) for the dichotomous variables. We compared the demographics and vascular risk factors in the stroke patients with and without CMB using a Student's t-test (for the continuous variables) and the Chi-square test (for the proportions). The multivariate analyses were performed by the inclusion of the prespecified factors that were associated with CMB in the univariate analysis. The study was approved by the Institute's Ethical Review Committee.
RESULTS
There were 200 patients in the study. Baseline characteristics of the patients are presented in Table 1. Mean age of the patients was 61-year-old. Mean NIHSS was 8. The prevalence of CMBs was 20% (39/200 patients). Intravenous recombinant tissue plasminogen activator was given in 83 patients (42%). MRI/MRA was performed in average 18 days after stroke onset. ICH and hemorrhagic transformation were found in 12 (6%) and 41 (21%) patients, respectively.
In the subgroup of patients who received intravenous thrombolysis (83 patients), hemorrhagic transformation and ICH did not occur more frequently in patients with CMB than in those without CMB (23.5% vs. 31.8%, P = 0.512). In patients with confluent white matter lesions (Fazekas 3), CMBs were found in 16 patients (43%) and located at cortico-subcortical area (6 patients, 37.5%), infratentorial area (4 patients, 25%), and both cortico-subcortical and deep cerebral hemisphere (6 patients, 37.5%).
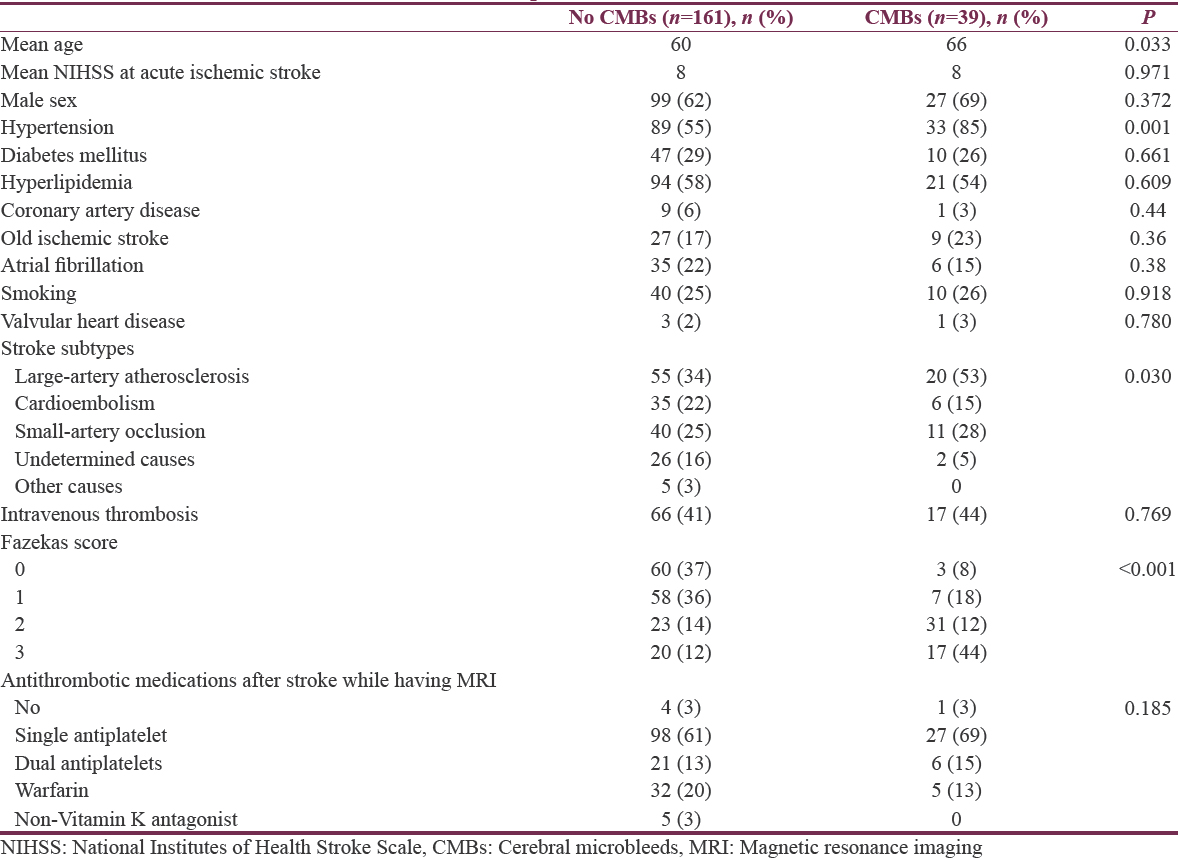
CMBs were related to older age, hypertension, some stroke subtypes (LAA, SAO), and higher Fazekas scale [Table 2]. However, multivariate analysis showed that only hypertension (OR 3.05, 95% CI 1.07–8.68, P = 0.037) and moderate-to-severe white matter lesions (Fazekas 2–3, OR 7.61, 95% CI 3.06–18.95, P < 0.001) were related to the presence of CMBs.
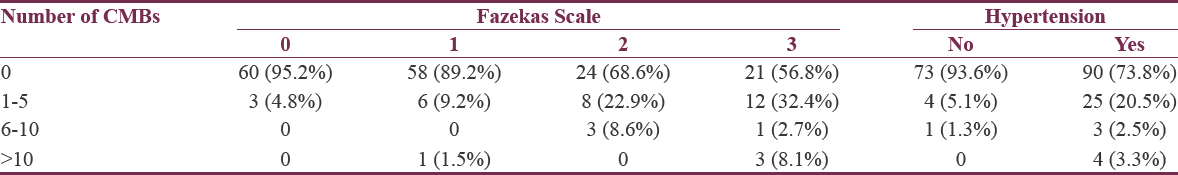
There was association between the number of CMBs and severity of white matter lesions or the presence of hypertension as higher number of CMBs related to more severe white matter lesions (P < 0.001) and the presence of hypertension (P = 0.001) [Table 3].
In a subgroup of patients who were on single antiplatelet, there was no significant difference in the presence of CMB and type of antiplatelet (aspirin; 23% (27/116) vs. clopidogrel; 20% (2/10), P = 0.495).
DISCUSSION
The prevalence of CMB was 20% in this study, which was lower than those reports from Japanese studies but closed a report from a Chinese study. The prevalence of CMBs in patients with ischemic stroke varies widely (0%–78%).[4] Ethnicity seems to be significant with higher prevalence of CMBs among participants of Asian origin (43%) compared with Western participants.[4] However, most studies in these reports came from studies in Japan.[89] CMBs were found in 24% of 458 Chinese patients with ischemic stroke in a study.[10]
CMBs were related to older age, hypertension, stroke subtypes (LAA, SAO), and leukoaraiosis in our study and in other studies. The finding that the prevalence of CMBs increased with age was reported in several studies in adult participants without stroke.[1112] In patients with ischemic stroke, CMBs were found in some stroke subtypes. Kato et al. studied 213 patients with stroke and reported higher prevalence of CMBs in patients with lacunar infarct (62%) than those with cardioembolic infarct (30%) and atherothrombotic infarct (21%).[8] Another study from Japan found that CMBs were associated with atherothrombotic (5/22, 23%) and lacunar (7/31, 23%) but not in cardioembolic stroke (0/13, 0%).[13] In our study, clinical characteristics of the patients, results of cardiac investigations and MRA were used to classify stroke subtypes. CMBs were found in patients with LAA (53%) and SAO (28%) more than those with CE (15%). Accumulation of ischemic injuries in brain parenchyma is thought to differ in atherothrombotic stroke and cardioembolic stroke, suggesting that the relationship of CMBs and stroke subtype in turn may reflect a different degree of fragility of vascular walls.[1415]
Our study revealed that severe white matter lesions (Fazekas 3) were related to the presence of CMBs. Strong associations of CMBs with confluent white matter lesions, leukoaraiosis, and lacunes were reported, all of which are known to be associated with chronic hypertension.[481315] The severity of white matter lesions was associated with the number of CMBs.[813] CMB may be an indicator for the severity of cerebral microangiopathy. Abnormal permeability of the arteriolar blood–brain barrier leading to extravasation of blood components has been suggested.[4]
The association of antithrombotic treatment and prevalence of CMBs is still debated as some studies showed the relationship but not in others.[4] Our study did not show a significant difference between antithrombotic uses in patients with or without CMBs. Although no patient receiving novel oral anticoagulation (NOAC) had CMBs, only a small number of patients used NOAC in the study. However, all MRI studies were performed after acute ischemic stroke, so most patients were on antithrombotic drugs. This may not accurately investigate the effect of antithrombotic medications on the presence of CMBs.
Charidimou et al. performed a systemic review and meta-analysis to assess whether the presence of CMBs on pretreatment MRI scans in patients with acute ischemic stroke treated with thrombolysis is associated with the increased risk of symptomatic ICH.[5] In this meta-analysis, the prevalence of CMBs was 23.3%. Among patients with CMBs, symptomatic ICH after thrombolysis was higher compared to those without CMBs (8.5% vs. 3.9%, OR2.26, 95% CI 1.46–3.49, P < 0.0001). In our study, although hemorrhagic transformation occurred more often in patients after intravenous thrombolysis as compared to those not having thrombolytic treatment (30% vs. 13.7%), the hemorrhagic transformation rate was not different in patients with or without CMBs (23.5% vs. 31.8%, P = 0.512). Most hemorrhagic transformation was asymptomatic, and only three cases were symptomatic.
As for limitations of this study, this was a retrospective study. Patients with ischemic stroke were included in the study if they had MRI/MRA during the study period. Most patients did not have pretreatment MRI, so the effect of antithrombotic drugs on the incidence of CMB could not be answered. However, this was the first study looking for CMBs in Thai patients with ischemic stroke. CMBs did not appear to be as high as previous Asian reports.
CONCLUSIONS
In our study, CMBs were found in 20% of Thai patients with ischemic stroke. CMBs were associated with hypertension and severity of the white matter lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- An up-to-date review on cerebral microbleeds. J Stroke Cerebrovasc Dis. 2016;25:1301-6.
- [Google Scholar]
- Cerebral microbleeds in the population based AGES-Reykjavik study: Prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002-6.
- [Google Scholar]
- Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology. 2008;70:1208-14.
- [Google Scholar]
- Cerebral microbleeds on MRI: Prevalence, associations, and potential clinical implications. Neurology. 2006;66:165-71.
- [Google Scholar]
- Cerebral microbleeds and postthrombolysis intracerebral hemorrhage risk Updated meta-analysis. Neurology. 2015;85:927-4.
- [Google Scholar]
- MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351-6.
- [Google Scholar]
- Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: The LADIS study. Arch Intern Med. 2007;167:81-8.
- [Google Scholar]
- Silent cerebral microbleeds on T2*-weighted MRI: Correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536-40.
- [Google Scholar]
- Assessment of lacunar hemorrhage associated with hypertensive stroke by echo-planar gradient-echo T2*-weighted MRI. Stroke. 2000;31:1646-50.
- [Google Scholar]
- Cerebral microbleeds and quality of life in acute ischemic stroke. Neurol Sci. 2011;32:449-54.
- [Google Scholar]
- MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991-4.
- [Google Scholar]
- Cerebral microbleeds: Prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831-5.
- [Google Scholar]
- Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: Association with combination of stroke subtypes and leukoaraiosis. AJNR Am J Neuroradiol. 2004;25:714-9.
- [Google Scholar]
- Low level of low-density lipoprotein cholesterol increases hemorrhagic transformation in large artery atherothrombosis but not in cardioembolism. Stroke. 2009;40:1627-32.
- [Google Scholar]
- Cerebral microbleeds: Their associated factors, radiologic findings, and clinical implications. J Stroke. 2013;15:153-63.
- [Google Scholar]






