Translate this page into:
Cerebral microbleeds: Causes, clinical relevance, and imaging approach – A narrative review
*Corresponding author: Amit Agarwal, Department of Radiology, Mayo Clinic, Jacksonville, United States. amitmamc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agarwal A, Ajmera P, Sharma P, Kanekar S. Cerebral microbleeds: Causes, clinical relevance, and imaging approach – A narrative review. J Neurosci Rural Pract. 2024;15:169-81. doi: 10.25259/JNRP_351_2023
Abstract
With advances in magnetic resonance imaging (MRI) sequences, there has been increased identification of microbleed/microhemorrhage across different population ages, but more commonly in the older age group. These are defined as focal areas of signal loss on gradient echo MRI sequences (T2* and susceptibility-weighted images), which are usually <5 mm in size representing hemosiderin deposition with wide ranges of etiologies. Susceptibility-weighted imaging (SWI) has become a routine MRI sequence for practices across the globe resulting in better identification of these entities. Over the past decade, there has been a better understanding of the clinical significance of microbleeds including their prognostic value in ischemic and hemorrhagic stroke. Cerebral amyloid angiopathy and hypertension are the two most common causes of microbleeds following peripheral and central pattern, respectively. In the younger age group, microbleeds are more common due to familial conditions or a wide range of hypercoagulable states. This review outlines the pathophysiology, prevalence, and clinical implications of cerebral microhemorrhage along with a brief discussion about the technical considerations of SWI.
Keywords
Cerebral
Microbleed
Hemorrhage
Magnetic resonance imaging
Susceptibility
INTRODUCTION
Cerebral micro bleeds (CMBs), also known as cerebral micro hemorrhages are small, rounded, hypointense foci <5 mm with sizes ranging between 5 and 10 mm detected at T2* sequences or susceptibility-weighted imaging (SWI). The histopathological evaluation of these lesions reveals that the CMBs are focal deposits of hemosiderin-laden macrophages. The hemosiderin imparts these small lesions paramagnetic properties causing them to lose signal due to susceptibility.[1,2] According to the Rotterdam Scan report, the average incidence in the general population over a three-year period was nearly 10%. The Rotterdam scan study found CMBs in about 40% of participants over 80 years of age. The overall incidence is also greatly distorted with diseased people much more likely than healthy people to display CMBs on imaging.[3]
Over the previous decade, there have been significant improvements in the strength of magnetic resonance (MR) systems moving from 1.5 T to 3 T and the recent leap to 7 T resulting in higher detection of CMBs. These are more frequent in the older population and their prevalence increases with age. Despite the significant increase in encountering CMBs, there is a lack of understanding in terms of their association with various clinical pathologies and expected imaging findings. The presence of CMB increases the likelihood of encountering cerebral strokes with more of them culminating in death; the association also affects the chances of hemorrhagic conversion of stroke.[4] The ability of MR imaging (MRI) to localize micro bleeds is dependent on various MR factors. Therefore, it becomes imperative to understand the factors affecting the optimal imaging of CMBs. Followed by this, we have discussed the association of CMBs with various pathologies and how they affect management.
TECHNICAL FACTORS
Pulse sequence, spatial resolution, time of echo, slice thickness, magnetic field intensity, and flip angle are some parameters known to affect CMB detection. The rate of encountering CMBs increases as the spatial resolution and slice thickness are increased, as this reduces the impact of partial volume effects and allows for the identification of smaller, more conspicuous CMBs.
Magnetic susceptibility is a characteristic of tissues that indicates a substance’s magnetic response to an external magnetic field. Local magnetic field inhomogeneity is caused by differences in susceptibility between substances, which causes faster T2* relaxation and signal loss in MR sequences receptive to T2* effect. When detecting blood elements, the T2*- Gradient-recalled echo (GRE) sequence outperforms the T2-weighted echo-spin sequence. The parameters of MRI affect the extent of the “blooming effect.” A prolonged duration of echo can detect (and magnify) more microbleeds than a short echo time allowing for increased dephasing of the focal MR signal. The SWI is a three-dimensional, flow-compensated, and radiofrequency spoiled GRE sequence based on tissue susceptibility differences. The SWI amalgamates magnitude and phase images (Pha) to assess these variations.
The SWI is more vulnerable to hemorrhage, calcium, iron storage, and slow venous blood than T2* GE sequences allowing for a significant transition. According to the main underlying principle, signal loss on SWI is in direct relation in the tissue to the presence of electrons that are not paired. As a result, the paramagnetic properties of deoxyhemoglobin in which the iron atom produces four unpaired electrons are used to visualize the cerebral veins [Figure 1a]. In subacute hemorrhage, SWI makes use of methemoglobin’s paramagnetic properties where the atom of iron exists in the ferric oxidation state and releases five unpaired electrons [Figure 1b]. The T2* signal degradation seen by SWI in old hemorrhage lesions is due to hemosiderin’s superparamagnetic properties including many unpaired electrons [Figure 1c]. The magnetic character and the stage of thrombosis have been determined as a correlation [Table 1].[5]
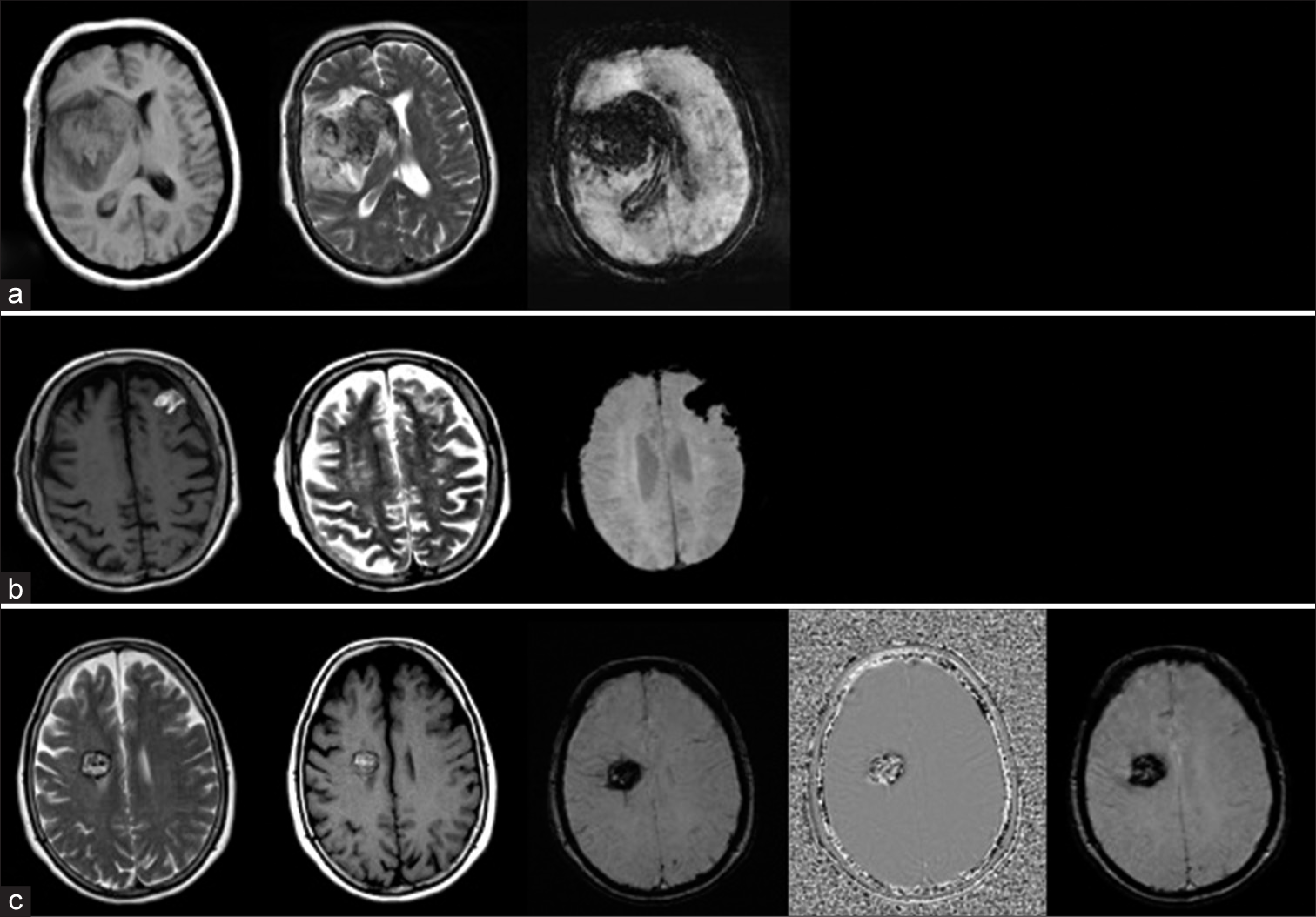
- Axial sections of magnetic resonance imaging revealing the appearance of cortical parenchyma due to (a) deoxyhemoglobin, (b) methemoglobin, and (c) super-paramagnetic hemosiderin.
| Stage of thrombosis & approximate duration | Status of hemoglobin |
Magnetism | Tl shortening (Hyperintense) |
T2 & T2* Shortening (Hypointense) |
|---|---|---|---|---|
| Acute (0-3 days) | DeoxyHb | T2 Paramagnetic |
__ | +++ |
| Early subacute (<l week) |
Intracellular MetHb |
T1 &T2 Paramagnetic |
+++ | ++++ |
| Late subacute (1-2 weeks) |
Extracellular MetHb |
T1 Paramagnetic |
++++ | __ |
| Chronic (>2 weeks) | Hemosiderin | T2 Paramagnetic |
__ | ++++ |
Generating an SWI image
This entails several steps that must be completed in a specific order, and the first is the filtration of Pha to reduce unnecessary susceptibility in the context. The filtered Pha produce a phase mask in the next step. As the next step, the phase mask is multiplied by the original magnitude picture resulting in amplification of the susceptibility effects. The final processing step involves stacking multiple SWI images to create a thick minimum intensity pixel (MIP). This requires at least four images being combined into a single MIP image [Figures 2 and 3]; this last step has a multiplicative effect on enhancing the susceptibility effects.

- Illustration of the process of generating a susceptibility-weighted image (SWI) from the magnitude and phase images.
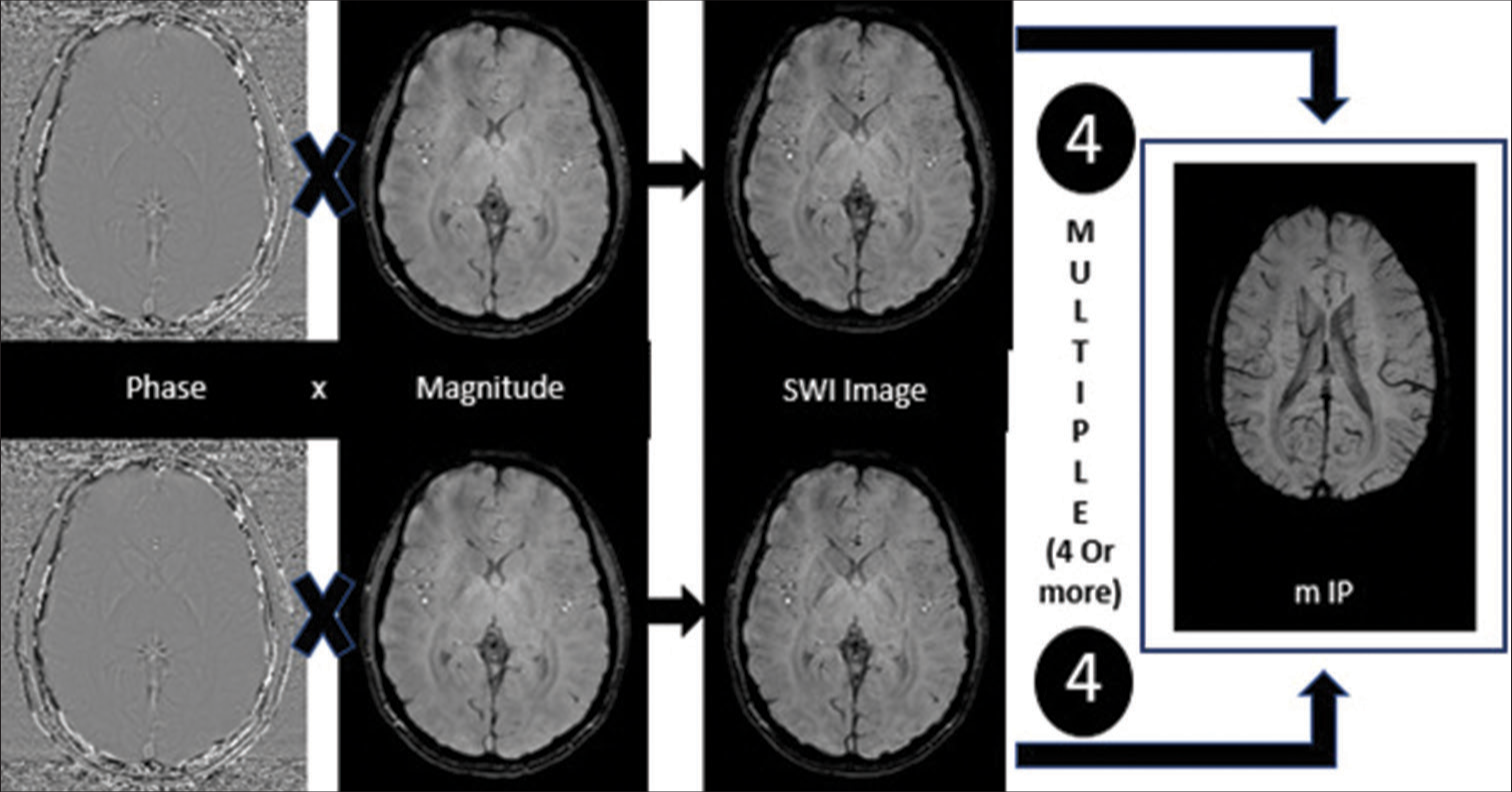
- Illustration of the process of generating a minimum intensity pixel image from the susceptibility-weighted images (SWI) produced in Figure 2. mIP: Minimum intensity projection
How to differentiate microcalcification from microhemorrhage on SWI
Due to local tissue susceptibility effects, SWI is the tool to detect calcium and iron deposits in white and gray matter using high-pass-filtered Pha. Calcium is diamagnetic and does not contain any unpaired electrons. However, it has low local field inhomogeneity causing phase shifts. Calcium causes a negative in-phase phase shift (in left-handed MR systems), which makes calcium appear hypointense on Pha [Figure 4a]. Using the filtered Pha, SWI can detect non-heme iron accumulation mainly in ferritin and transferrin. The paramagnetic non-heme iron has a positive phase shift and appears hyperintense on Pha [Figure 4b].
![Axial computed tomography (CT) and magnetic resonance imaging (susceptibility-weighted imaging [SWI] and Phase) images in two separate patients reveal hyperdensity on CT images and foci of blooming in SWI in both patients. Phase sequence reveals (a) hypointensity in the first patient due to calcium and (b) hyperintensity in the other patient due to non-heme iron. Note: The phase description is applicable to left-handed magnetic resonance scanners.](/content/150/2024/15/2/img/JNRP-15-169-g004.png)
- Axial computed tomography (CT) and magnetic resonance imaging (susceptibility-weighted imaging [SWI] and Phase) images in two separate patients reveal hyperdensity on CT images and foci of blooming in SWI in both patients. Phase sequence reveals (a) hypointensity in the first patient due to calcium and (b) hyperintensity in the other patient due to non-heme iron. Note: The phase description is applicable to left-handed magnetic resonance scanners.
7T versus 3T versus 1.5T MRI: How valid is the argument?
Theoretically, with an increase in susceptibility effects and signal-to-noise ratio with increasing strengths of field, there should be an increase in the detection of CMBs. Various studies performed to date have also proven this practically, especially when comparing 3T with 1.5T. The jump from 3T to 7T, the real-life difference in detection rate, remains to be proven. While logic dictates that with a further increase in sensitivity, there will be a corresponding increase in CMB diagnosis, the increased sensitivity is a double-edged sword, as the heightened susceptibility can obstruct the diagnosis of CMBs in regions likely to have increased susceptibility effects.
Multiple studies have suggested that high field strengths allow for shorter TEs without compromising the quality of the image acquired. Shorter echo times at 7T in adjunct with improved resolution permit the best detection rate among all sequences.[6,7]
CAUSES OF CMBs
The various pathology of CMBs can be classified into broad categories [Table 2] for easier understanding. This includes sporadic small vessel disease as the largest category, especially in the older age group featuring cerebral amyloid angiopathy (CAA) and hypertensive angiopathy as the leading causes. Small-vessel disease secondary to neuroinflammatory conditions is the other big category characterized primarily by small-vessel inflammation with resultant leakage and microhemorrhage. Neurodegenerative conditions are increasingly being recognized by the robust increase in our understanding of molecular markers. Other etiologies include diffuse-axonal injury, post-radiation changes, encephalitis, and infections.
| Pathology | Small vessel disease associated with inflammatory, immunologically mediated, and hematological disorders |
Sporadic cerebral small vessel disease | Inherited or genetic small vessel disease | Other conditions |
| Disease | 1. SLE 2. Sickle cell Disease 3. DIC 4. ITP S. Sneddon syndrome |
1.Sporadic CAA 2.Hypertensive arteriolopathy |
1. CARASIL CADASIL Fabry disease Hereditary CAA Small vessel disease caused by COL4Al mutation |
1.Post-radiation/post-treatment 2. Traumatic brain injury 3.PRES 4.Other encephalopathies 5. Moya-mayo |
SLE: Systemic lupus erythematosus, DIC: Disseminated intravascular coagulation, ITP: Immune thrombocytopenia, CAA: Cerebral amyloid angiopathy, CARASIL: Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy, CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, COL4Al: Collagen alpha-1(IV), PRES: Posterior reversible encephalopathy syndrome
Sporadic cerebral small-vessel diseases
Hypertensive arteriopathy (HA) and CAA are two of the most common causes of CMBs [Figure 5]. Both are associated with hemorrhagic and non-hemorrhagic parenchymal changes such as CMBs, cortical superficial siderosis, white matter hyperintensities, and, more recently, MRI-visible expanded perivascular spaces. One of the most important distinctions between these two is the location of predilection which is lobar in CAA and deep arteriolar in HA. The HA (lipohyalinosis and arteriolosclerosis) is a condition that affects the deep gray nuclei and deep white matter’s small perforating end-arteries. Hypertensive microbleeds are characteristically seen in deep brain structures (basal ganglia, thalamus, and brainstem). It is also important to note that this can coexist with CAA-related changes making the segregation of microbleeds difficult.[8,9]
- (a) Susceptibility-weighted images demonstrating cerebral microbleeds in the deep brain regions including basal ganglia, thalamus, and brainstem (b) in a case of hypertensive arteriopathy; peripheral lobar distribution in a patient with cerebral amyloid angiopathy.
CAA
The CAA is a condition in which there is the deposition of protein in the tunica media and adventitia of the vascular layers of small arteries, arterioles, and capillaries in the cerebral cortex, overlying meninges and the gray-white matter junction. The most common protein in the sporadic CAA is amyloid-β, an eosinophilic, insoluble protein of 42 amino acid peptides cleaved from amyloid precursor protein on chromosome 21. This accumulation of proteins results in fibrinoid degeneration in the tunica media and tunica intima leading to microaneurysm formation. Imaging studies and histological staining of the biopsied tissues are used to establish a diagnosis. On computed tomography (CT), this is characterized by lobar pattern parenchymal hematomas. On MRI, the appearance of hemorrhage will vary according to the age of the bleed. The CMBs in a lobar distribution (cortical-subcortical) characterize CAA. To confirm the diagnosis, a biopsy is done with Congo staining, which shows apple-green birefringence when viewed with polarized light. When stained with thioflavin T, it illuminates with ultraviolet light, and the AB deposits emit bright green fluorescence. In older populations, CAA and HA can coexist resulting in a “mixed” lobar and deep distribution of CMBs, which poses a diagnostic challenge.[10]
Luminal stenosis and fibrinoid necrosis are caused by the deposition of homogeneous eosinophilic deposits in vessels of the cortex and meninges. The vessels become weaker and more susceptible to bleeding. There is no direct connection between CAA and high blood pressure, diabetes or atherosclerosis. On imaging, surface lobar hematomas with subcortical or subarachnoid extension are visible. The 70% of patients have non-hemorrhagic diffuse encephalopathy and focal or patchy/confluent white matter (WM) disease. In CAA patients with multiple lesions, the distribution of CMBs shows a cortical predominance posteriorly, and they often appear to group in the same lobe. Blood-sensitive MRI sequences are also critical for detecting sporadic CAA markers such as superficial cortical siderosis and convexity subarachnoid hemorrhage (SAH) [Figures 6 and 7]. Apart from the already known lobar position in CAA-related intracerebral hemorrhage (ICH), studies have reported that CAA-related parenchymal hemorrhages are more likely to have subarachnoid space extension, an irregular ICH boundary, and multiple recurrent episodes of hemorrhage. A proposal for a classification scheme based on CT and genetic makeup as well as Edinburgh CT and genetic diagnostic criteria was recently developed. The presence of an ApoE 4 genotype and CT findings such as SAH and finger-like projections of the hematoma border were used to make the diagnosis.[9,10] There are no specific treatment modalities available for CAA. It is seen that antihypertensive medications prevent ICH recurrence whereas antithrombotic may increase hemorrhage risk. There is a huge burden of CAA in elderly patients, which is unrecognized. Therefore, research efforts to develop or discover biomarkers for early detection of CAA can reduce the cases of microbleeds (MCB) and simultaneously give opportunities to develop treatment interventions.[11,12]
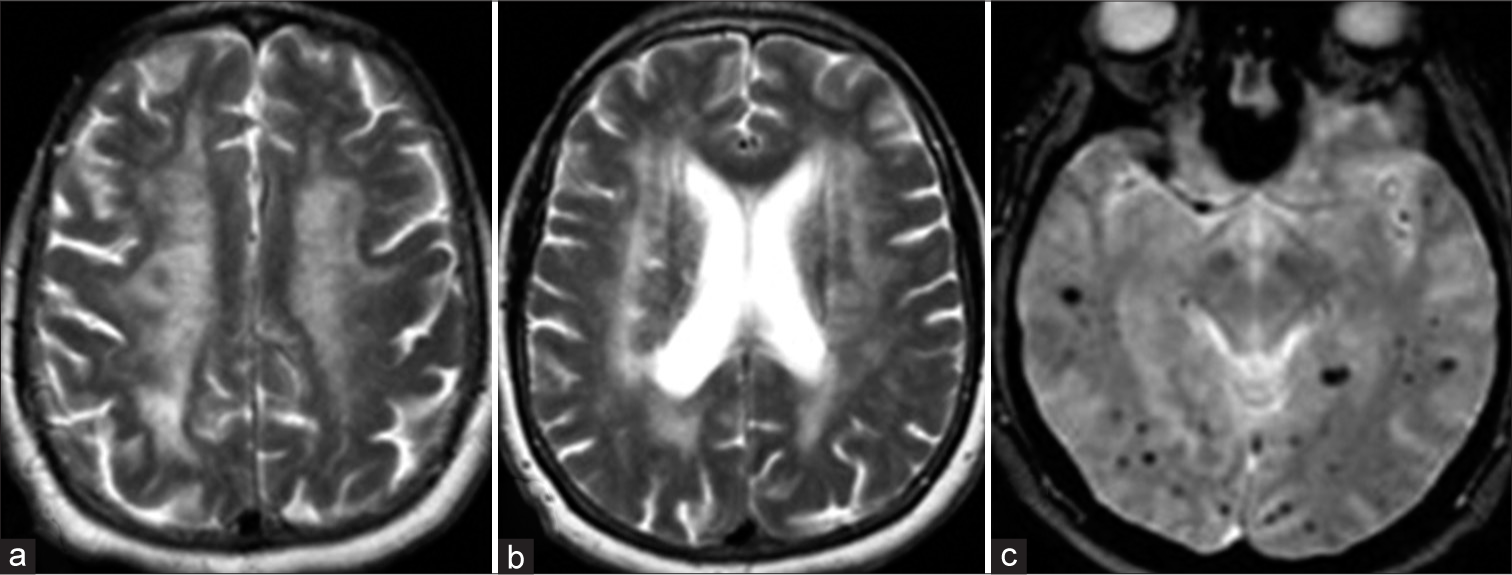
- (a and b) Axial T2WI and (c) susceptibility-weighted imaging sequences in a 71-year-old male patient who presented with cognitive decline showing classical imaging findings of amyloidangiopathy (white matter changes with subcortical microhemorrhages).

- (a and b) Axial T2*- and (c) T1-weighted sequences in a 76-year-old male patient with amyloid angiopathy who presented with acute head and weakness due to right parietal hemorrhage.
HA
High arterial blood pressure is one of the most common causes of vascular diseases. High arterial blood pressure primarily affects the small arteries in the brain, which results in these arteries undergoing segmental constriction in response to persistent high blood pressure. The long-term constriction leads to smooth muscle hypertrophy in the tunica media by collagen fibers. Chronic arterial hypertension brings structural changes in the intraparenchymal arteries and arterioles called arteriolosclerosis including microadenoma, lipohyalinosis, fibroid necrosis, and microaneurysm (Charcot Bouchard aneurysm). The most common sites for HA are basal ganglia, thalamus, cerebellum, and pons, small vessels prone to hypertensive-induced vascular injuries.
Patients who suffered from microhemorrhaging were at the extreme of old age and had an elevated risk of hypertension. A link was established between the number of microhemorrhages and white matter hyperintensity. Hypertension is among the most common causes of diseases of the small vessel most commonly presenting as non-specific white matter signal changes on imaging representing damage to tissue caused by microangiopathy. The classic location for microhemorrhages includes the lentiform nucleus, thalamus, and brainstem, which are all common sites for symptomatic hematomas. An MRI in hypertensive microangiopathy reveals white matter T2 hyperintensities, a variable number of microbleeds seen best on GRE or SWI, in addition to ICH. Treatment in such patients is to keep the blood pressure under control.[4,13,14]
Neuroinflammatory and hematological disorders
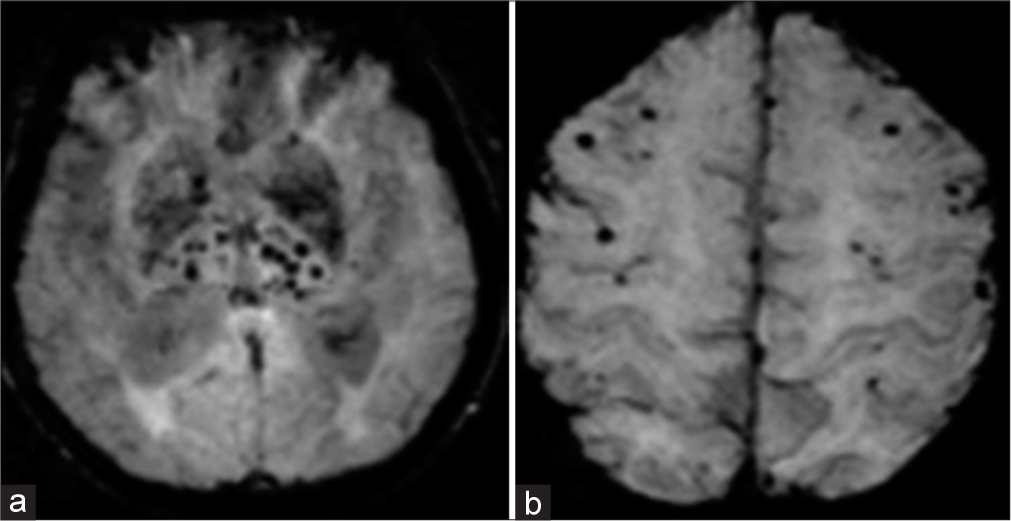
Hemorrhagic or ischemic stroke can be caused by a variety of neuroinflammatory and hematological disorders. Abnormal blood counts, vessel wall damage or a combination may cause this increase the risk for stroke. In patients with leukemia and thrombocytopenia, intracranial bleeds are common due to coagulation factor intake or the disease’s blastic phase [Figures 8 and 9]. According to estimates, central nervous system complications affect 2–4% of leukemia patients per month. A mass lesion within neural tissue, tumor cell invasion of neural tissue or pressure from outside on neural tissue by an extrinsic mass, straightaway involving the blood vessels, may all play a role in leukemia.[15,16]
- (a and b) Axial susceptibility-weighted imaging images reveal multiple tiny hemorrhages in the cerebral parenchyma during the indolent phase of acute lymphoblastic leukemia, while the computed tomography scan was negative for any bleeding.

- (a) Axial susceptibility-weighted imaging images, (b) fluid attenuation inversion recovery, and (c) computed tomography reveal multiple tiny hemorrhages in the cerebral parenchyma during the blast crisis of acute lymphoblastic leukemia (leukocyte>300,000/mm3).
Critical illness-associated CMBs
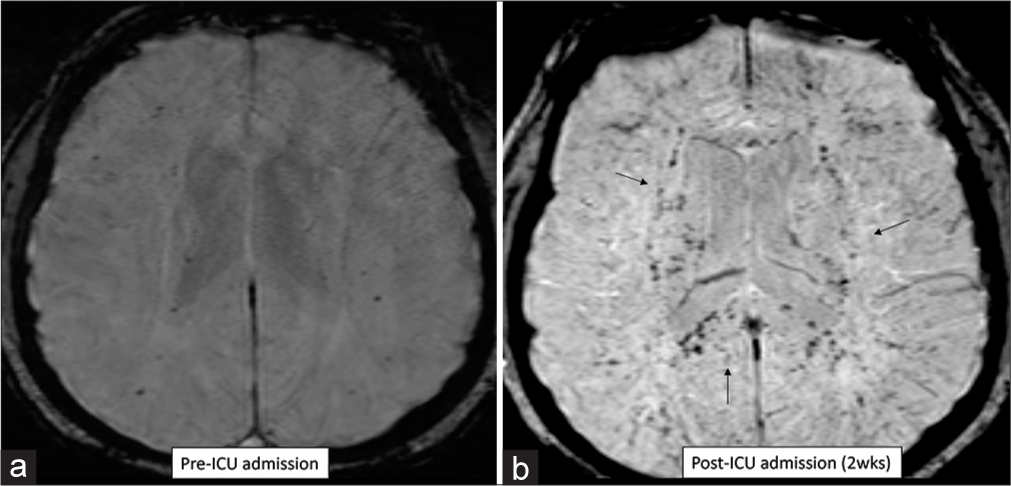
Critical illness associated microbleeds is a newly described term encompassing many underlying etiologies and primarily seen in intensive care unit (ICU) patients. There is diffuse involvement of the juxtacortical white matter and corpus callosum with sparing of the cortex and periventricular regions [Figure 10].[17-19] Extensive involvement of the corpus callosum is a hallmark of this entity, more commonly seen in the younger age group.[20] Respiratory failure is a leading cause for this entity, typically in an ICU setting secondary to acute respiratory distress syndrome, lung transplant, and cystic fibrosis. Other common etiologies include leukemia, lymphoma, sickle cell disease encephalitis, and H1N1 influenza infection. Regardless of the underlying etiology, acute respiratory failure is a common feature in all reported patients as well as our patients. The CMBs can develop rapidly over the course of a few days to weeks and usually remain stable over subsequent follow-up scans.[20,21]

- (a and b) Susceptibility-weighted imaging images in a patient with respiratory failure, pre- and post-intensive care unit (ICU) admission. Numerous microbleeds noted in the brain, involving the corpus callosum, 2 weeks after admission to ICU. Findings consistent with “critical-illness related microbleeds.”
Thrombocytopenia
Thrombocytopenia is a condition where the platelet count is <150,000 platelets per microliter of circulating blood. The primary role of platelets is to maintain hemostasis and take part in angiogenesis and innate immunity. A platelet count as low as 80 × 109/L may be appropriate for hemostasis if platelet function is normal. When the platelet count falls below 20 × 109/L, clinically important spontaneous hemorrhage occurs. Anemia, leukemia, disseminated intravascular coagulation (DIC), or drug-induced thrombocytopenia (quinine, quinidine, gold salts, phenytoin, valproic acid, carbamazepine, sulfonamides, and heparin-induced) are all potential causes of thrombocytopenia. Immune thrombocytopenia (ITP) is an autoimmune disorder associated with an aberrant immune response resulting in thrombocytopenia. Spontaneous microbleeds can frequently be seen in patients with platelet counts <30 × 109. CMBs occur more in ITP with longer duration and lower nadir platelet counts.[22]
DIC
This syndrome is caused by the loss of clotting factors and platelets, faulty and stimulated fibrinolysis, and a deterioration of the coagulation inhibition mechanism. Fibrin-rich thrombi are seen pathologically in arterioles, capillaries, and venules. The cause of mortality in DIC patients is multi-organ dysfunction and severe bleeding. DIC is not a separate condition but a symptom of an underlying disorder or disorder. Infections, malignancies, obstetric diseases, trauma, blood disorders, aneurysms, and liver disease can complicate DIC. DIC symptoms and signs affecting the nervous system are linked to thrombotic and hemorrhagic complications. There are different treatment modalities to treat DIC along with treating the underlying conditions, including blood transfusion in massive bleeding, heparin in non-symptomatic DIC, synthetic proteases inhibitor, and antifibrinolytic in symptomatic DIC, natural proteases inhibitors in organ failure DIC.[22,23]
Neurodegenerative diseases
It is a condition established by progressive nervous system degeneration, which results in gradual loss of cognition, motor skills, and overall neurological abilities. Deposition of iron is increased in neurodegenerative diseases, including Parkinson’s, Huntington’s, Alzheimer’s, multiple sclerosis, and pantothenate kinase-mediated neurodegeneration. The ability to quantify the magnitude of iron from sources other than heme in the cortex will help researchers better understand disease symptoms’ pathogenesis and progression and predict the treatment outcomes. Lobar CMBs have been discovered in more than 20% of patients with intermittent Alzheimer’s disease (AD) (ranging from 15% to 32%). In dementia such as frontotemporal dementia, corticobasal degeneration, dementia with Lewy bodies, and progressive supranuclear palsy, CMBs are much less common. Their distribution indicates that in Alzheimer’s disease, lobar CMBs reflect the underlying CAA.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)/cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL)
A mutation in the NOTCH3 gene on chromosome 19q12 causes CADASIL, an inherited disease. Progressive memory decline, migraines with aura, mood swings, and small vessel infarcts are common in patients. MRI features include changes in white matter on T2-weighted images, lacunar infarcts on T1-weighted images, and CMBs. Microbleeds have been reported to occur in 25–70% of cases of CADASIL without lobar preference. White matter differences in a periventricular distribution affect the anterior temporal lobes, corpus callosum, and external capsule. The capsular-striatal zone, the thalami, and the central pons are popular sites for lacunar infarcts. Cognitive function loss is linked to CMB burden and position, lacunar infarcts, and atrophy of the cortex.[24] CARASIL is a systemic genetic disorder affecting small intracranial vessels, spine, and hair follicles. This usually presents in the second or third decade of life with premature alopecia, relapsing ischemic stroke-like episodes and epilepsy. The exact incidence of microbleeds in CARASIL is poorly documented at present, given the rarity of this condition.[24,25]
Traumatic brain injury (diffuse axonal injury)
CMBs result from diffuse axonal damage in the form of traumatic brain injury arising after sudden rotational, acceleration, and deceleration injuries. Due to intermittent small-vessel disease and other imaging characteristics of brain damage, traumatic microbleeds can normally be distinguished from CMBs. Traumatic microbleeds, on the other hand, vary from other CMBs in that they typically occur at the junction of gray-white matter in the frontal and temporal lobes, brainstem, and corpus callosum. The seriousness of axonal injury has been linked to a poor prognosis. For hemorrhagic and non-hemorrhagic DAI, MRI is much more sensitive than CT in detecting and classifying injury patterns. Importantly, it is common for CT to be normal or show subtle cerebral edema in DAI with a complete absence of hemorrhagic changes. Poor Glasgow coma scale in a patient with severe (level 1) trauma, should prompt an MRI study with SWI for detection of these microhemorrhages and better prognostication.[26]
Other conditions
Infective endocarditis
The inflammation of the innermost layer of the endocardium characterizes infective endocarditis. The pathophysiology of endocarditis can be infective (most commonly bacterial in nature) or non-infective. Complications of infective endocarditis include infective intracranial aneurysm, which leads to the dilation of an arterial wall due to the infection. This mycotic aneurysm leads to CMBs in infective endocarditis patients. Mycotic aneurysms are more prone in immune-compromised hosts such as diabetes mellitus, steroid users, HIV or malignancy/chemotherapy. CMBs are not unusual in the case of infective endocarditis acquired from the community, seen in around 57% in the endocarditis group compared with <20% in controls. CMBs can frequently be seen adjacent to the cerebral sulci, representing small bleeds secondary to tiny sulcal or subpial mycotic aneurysms [Figure 11]. There is currently no modality with an adequate spatial resolution to detect sub-millimeter aneurysms. Occasionally, these tiny mycotic aneurysms can mimic microbleeds; however, these are within the sulci instead of being parenchymal and, unlike other aneurysms, can spontaneously regress.[4,27]

- (a-c) 41-year-old female patient with infective endocarditis shows areas of microhemorrhages (red arrow) and a small old subarachnoid hemorrhage in the right frontal lobe (green arrows). The patient presented with the right-side weakness due to an acute infarct in the left parietal lobe (teal arrow) with low apparent diffusion coefficient value.
Atrial myxoma
Atrial myxoma is the most common primary cardiac tumor accounting for about 50% of cardiac tumors. It occurs 70% of the time in the left atrium, either at the mitral annulus or the fossa ovalis border of the interatrial septum. Myxoma cells are formed from the multipotent mesenchymal cells. A triad of complications, including obstruction, embolic, and constitutional symptoms (such as fever, weight loss, malaise, anorexia, and high erythrocyte sedimentation rate), is associated with atrial myxoma. The villous and friable myxoma has a high potential to cause embolic events, whereas smooth myxoma usually causes obstruction. A quarter of left atrial myxoma patients develop neurological events which most frequently occur due to ischemic stroke, which is secondary to embolization from the heart. The multiple embolizations cause multiple subcortical infarcts that produce dementia over many years. Other causes of neurological manifestations are aneurysms and pseudoaneurysms, formed by the tumor cell infiltrating the cerebral vessel wall, weakening the internal elastic lamina. The embolized particles from the myxoma tumor are believed to be responsible for the microbleed. Echocardiogram is the investigation of choice as it is quick and easily available [Figure 12]. Cardiac MRI is also a diagnostic test to detect cardiac myxoma and provides a fair idea about the microenvironment within the tumor in T1- and T2-weighted sequence. Surgical excision is the treatment of choice for cardiac myxoma.[28]
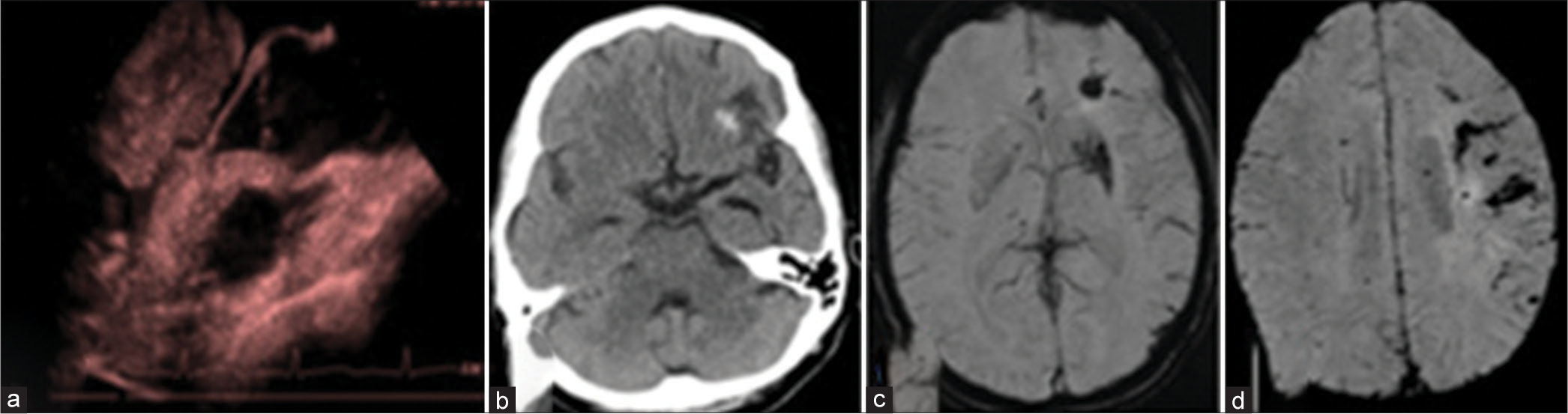
- (a) Cardiac Doppler shows large left atrial myxoma. (b) Computed tomography, and (c and d) magnetic resonance imaging performed for the same patient with complaints of acute severe headache shows acute left frontal bleed with the left frontal subarachnoid hemorrhage.
Moyamoya syndrome
The intracranial internal carotid arteries and their proximal branches become occluded in Moyamoya syndrome, a chronic progressive occlusive cerebrovascular condition. Reduced blood flow to the corresponding vessels leads to the formation of collateral vasculature. The cause of bleeding is associated with the rupture of fragile collateral vessels. On catheter angiography, the fine tortuous collateral vessels appear as a typical “puff of smoke.” CMBs were mostly found in the paraventricular zone, the subcortical white matter in the temporal lobe, and the basal ganglia [Figure 13]. The existence of multiple CMBs is related to an increased risk of ICH.[29]
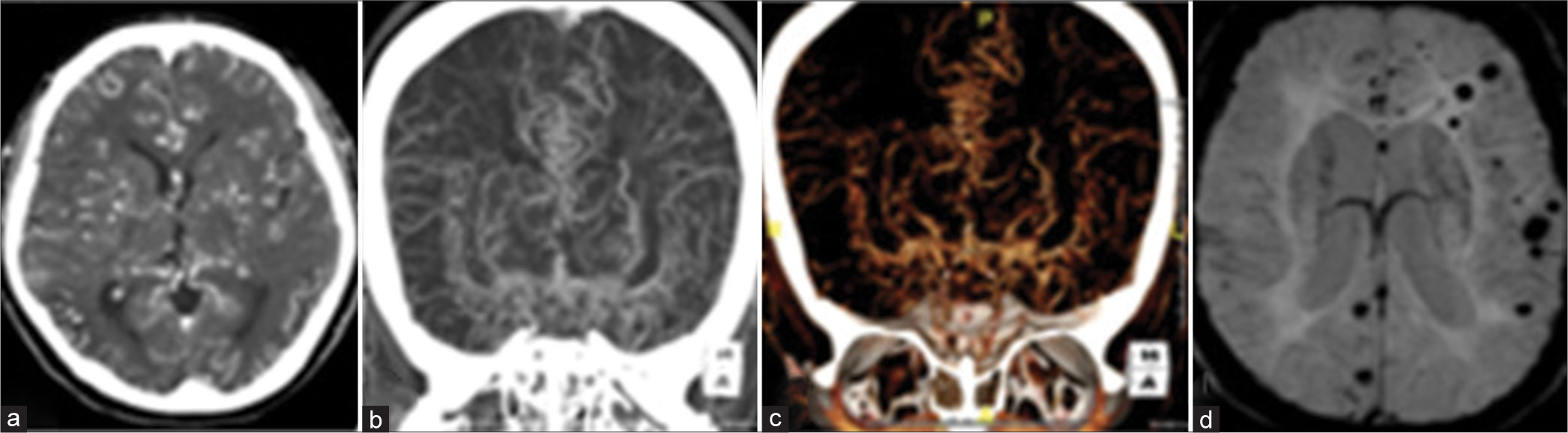
- (a-c) Computed tomography angiogram of a 41-year-old female patient reveals extensive collateral formation throughout the cerebral parenchyma with severe narrowing of the terminal internal carotid arteries. (d) Susceptibility-weighted imaging revealed multiple tiny chronic hemorrhages. The patient was diagnosed with primary Moyamoya disease.
Posterior reversible encephalopathy syndrome (PRES)
PRES is a neurologic disorder established when a person presents with visual disturbance, seizure, headaches, and altered mentation. It is believed that PRES syndrome has the following risk factors hypertension, preeclampsia, kidney diseases, autoimmune disorders (thrombotic thrombocytopenia purpura, eosinophilic granulomatosis with polyangiitis, and systemic lupus erythematosus), use of cyclosporine following organ transplantation, and sepsis. Hypertension is believed to be the main triggering factor for PRES syndrome. The PRES can occur acutely or subacutely within hours to days. In acute cases, it is seen that the blood pressure usually ranges from 160 to 190 mmHg. On MRI, fluid attenuation inversion recovery sequences reveal focal areas with symmetric hemispheric hyperintensities, most notably in the parietal and occipital lobes, indicating vasogenic edema. Hemorrhage was once thought to be an atypical characteristic of PRES, but microbleeds can be seen in 15–60% of subjects in whom SWI is acquired. The most accepted theory says that PRES in association with CMBs occurs due to endothelial cell dysfunction. For treatment purposes, removing the underlying causative agent, including controlling the blood pressure will reverse the edematous changes; however, microhemorrhages are irreversible. In acute cases, a sudden reduction of blood pressure can cause cerebral hypoperfusion of the brain and lead to brain ischemia; therefore, blood pressure should not drop more than 10–20 mm, every 10–20 min.[30]
Renal failure and microhemorrhages
The precise cause of CMBs in patients with renal failure is unclear. It is believed that chronic kidney disease (CKD) patients have an increased prevalence of microbleeds. The uremic toxin affects the blood-brain barrier permeability leading to microbleeds. MRI studies have shown a positive correlation between microbleeds in CKD patients with age-matched non-CKD control. Microhemorrhages have been reported to be more common in hemodialysis patients with chronic renal failure. Cho et al. examined 152 patients with a gradient-echo imaging scan and an acute ischemic stroke. They discovered a clear connection between inadequate kidney function and the existence of microbleeds. According to Ryu et al., CMBs are associated with chronic renal disease in patients who do not have diabetes, but not in diabetic patients with no renal disease. The uremic toxins affect the blood-brain barrier permeability leading to microbleeds. Many MRI studies have shown a positive correlation between microbleeds in CKD patients with age-matched non-CKD control.[31,32]
DIFFERENTIAL DIAGNOSIS OF MICROHEMORRHAGES
Prominent venous channels can mimic microbleeds on imaging; however, with experience and utilization of the MIP images, these can be segregated. Intracranial calcifications can be mistaken for microbleeds on MRI (SWI sequence), especially in the absence of any CT correlation. Calcifications, however, show low signal on all sequences (T1- and T2-weighted images), do not show blooming on SWI and with experience can be demarcated utilizing the phase and magnitude components of the SW images. A few other common differentials are outlined below:
Fat-emboli
Fat emboli is one of the leading differentials of microbleeds with similar imaging appearance on SWI. This is usually seen in patients with long bone fractures and during orthopedic prosthetic surgeries. The fat emboli can reach any organ in the body and most frequently involve the lungs, brain, and skin. Common clinical symptoms include petechial rash, pulmonary dysfunction, and neurological symptoms and usually develop —one to two days after the event. The foci of SWI drop-out seen in fat emboli are associated with numerous foci of diffusion restriction, which helps in demarcating this from microbleeds. The diffusion restriction in fat emboli is seen as numerous scattered hyperintense dots secondary to small infracts caused by blockage of the small arteries. This pattern is described as a “starfield” pattern and is a helpful differentiating imaging feature.[21]
Hemorrhagic metastasis
Metastatic hemorrhagic lesions from melanoma or renal cell carcinoma may mimic CMBs. To differentiate between these lesions and CMBs, we need a good clinical history and supportive imaging findings like perilesional edema. Perilesional edema is classically absent in microbleeds.[33]
Familial cavernous malformations
Clusters of dilated endothelial vessels with no intervening neural tissue form well-circumscribed nodules. The “popcorn appearance” is caused by the appearance of a rim of hemosiderin with a center of heterogeneous signal strength due to the hemorrhage of different chronological appearances. Small (Type-4) cavernomas appear as focal, hypointense lesions on T2* GRE images, which may be mistaken for CMBs.[34]
Radiation-induced cavernomas
Cavernomas are congenital in most cases, with a prevalence of up to 0.5%. Hyalinization and fibrinoid necrosis of small arterioles and arteriole walls, lumen narrowing, and endothelial proliferation have been identified as causes of radiation-induced cavernoma and capillary telangiectasia. Increased vascular endothelial growth factor expression was observed in an animal model of radiation-induced spinal injury. It may also cause mineralizing microangiopathy with dystrophic calcification, which is common in young children who have undergone chemotherapy or radiotherapy. Cavernomas have a very long latent period of development post-radiotherapy, running from 1 to 26 years.[35]
CLINICAL SIGNIFICANCE OF MICROBLEED
Cognition
The most common cause of vascular cognitive dysfunction is small vessel disease. The precise function of cognition in microbleeds, as well as the exact number, remains unknown. Experiments show that CMBs are not clinically “silent” when it comes to cognition. The correlation between CMBs and executive, pace, and attention dysfunction has been proven. Despite accounting for and calibrating for vascular risk factors and other imaging features of small vessel disease, multiple (>5) CMBs, particularly those occurring in solely lobar distribution, were linked to poor cognitive performance in the Rotterdam Scan Study.
Marker of repeated hemorrhage
The lobar CMBs in amyloid angiopathy patients predict an increased risk of persistent symptomatic ICH in the future.
Marker for recurrent stroke
An elevated risk of recurrence, mainly due to ischemic events instead of ICH, has been seen in subjects demonstrating CMBs, particularly the ones distributed in a mixed or lobar distribution but barring the deep CMBs.
Anticoagulation therapy and CMB
In patients needing long-term anticoagulant treatment, recognizing CMBs and excluding early changes of amyloid angiopathy is critical. With respect to their age-matched monitors, these patients have a higher risk of intracranial hemorrhage. As a result, it is reasonable to believe that accurate detection of CMBs (especially multiple lobar CMBs) would significantly impact treatment decisions in the future. In patients with CAA, a small prospective research group discovered that aspirin could be linked to the risk of recurrent lobar ICH.[1-4]
CONCLUSION
Microbleeds are becoming more commonplace on routine brain MRI due to the advancement in MRI techniques (GRE and susceptibility-weighted sequences). The incidence of CMBs is highest in the older age group secondary to hypertensive and CAA, followed by neurodegenerative diseases. These conditions, however, frequently co-exist. Microbleeds, once considered nonspecific and benign, are now linked to the prognosis of various neurological conditions, including the progress of neurodegenerative diseases and the tendency for hemorrhagic and ischemic stroke.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cerebral microbleeds: A guide to detection and clinical relevance in different disease settings. Neuroradiology. 2013;55:655-74.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165-74.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of cerebral microbleeds in the general population: The Rotterdam Scan Study. Stroke. 2011;42:656-61.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds: Imaging and clinical significance. Radiology. 2018;287:11-28.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612-8.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging detection of cerebral microbleeds: Effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338-43.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility-weighted imaging: Technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232-52.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19-30.
- [CrossRef] [PubMed] [Google Scholar]
- MRI of cerebral microhemorrhages. AJR Am J Roentgenol. 2007;189:720-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral amyloid angiopathy burden associated with leukoaraiosis: A positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013;73:529-36.
- [CrossRef] [PubMed] [Google Scholar]
- Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138:2126-39.
- [CrossRef] [PubMed] [Google Scholar]
- The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral hemorrhage associated with cerebral amyloid angiopathy: Model development and diagnostic test accuracy study. Lancet Neurol. 2018;17:232-40.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertension-related cerebral microbleeds. Case Rep Neurol. 2020;12:266-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology of hypertensive arteriopathy. Neurosurg Clin N Am. 1992;3:497-507.
- [CrossRef] [Google Scholar]
- Management of hemostatic complications in acute leukemia: Guidance from the SSC of the ISTH. J Thromb Haemost. 2020;18:3174-83.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and risk factors for central nervous system relapse in children and adolescents with acute lymphoblastic leukemia. Rev Bras Hematol Hemoter. 2012;34:436-41.
- [CrossRef] [PubMed] [Google Scholar]
- Critical illness-associated cerebral microbleeds for patients with severe COVID-19: Etiologic hypotheses. J Neurol. 2021;268:2676-84.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric critical illness associated cerebral microhemorrhages. eNeurologicalSci. 2019;18:100221.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple punctate cerebral hemorrhages in acute leukemia with blast crisis. Neurology. 2007;68:953.
- [CrossRef] [PubMed] [Google Scholar]
- Critical illness-associated cerebral microbleeds. Stroke. 2017;48:1085-7.
- [CrossRef] [PubMed] [Google Scholar]
- Critical illness-associated cerebral microbleed in a patient with sickle cell disease: A case report and review of the literature. Clin Imaging. 2020;68:184-7.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of occult cerebral micro-bleeds in adults with immune thrombocytopenia. Blood. 2020;136:2875-80.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of and risk factors for cerebral microbleeds among adult patients with haemophilia A or B. Haemophilia. 2018;24:271-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as a model of small vessel disease: Update on clinical, diagnostic, and management aspects. BMC Med. 2017;15:41.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: Diffuse cerebral microbleeds in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy. Front Neurol. 2022;13:818332.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurol Res. 2008;30:974-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds are frequent in infective endocarditis: A case-control study. Stroke. 2009;40:3461-5.
- [CrossRef] [PubMed] [Google Scholar]
- Scattered cerebral microbleeds due to cardiac myxoma. Arch Neurol. 2009;66:796-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of and risk factors for cerebral microbleeds in moyamoya disease and syndrome in the American population. Cerebrovasc Dis Extra. 2019;9:139-47.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:896-903.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology. 2009;73:1645-8.
- [CrossRef] [PubMed] [Google Scholar]
- The relation between chronic kidney disease and cerebral microbleeds: Difference between patients with and without diabetes. Int J Stroke. 2012;7:551-7.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical hemorrhagic brain metastases mimicking cerebral microbleeds. J Neurocrit Care. 2017;10:129-31.
- [CrossRef] [Google Scholar]
- Accuracy of SWI sequences compared to T2*-weighted gradient echo sequences in the detection of cerebral cavernous malformations in the familial form. Neuroradiol J. 2016;29:326-35.
- [CrossRef] [PubMed] [Google Scholar]
- Brain radiation-related black dots on susceptibility-weighted imaging. Neuroradiol J. 2014;27:445-51.
- [CrossRef] [PubMed] [Google Scholar]







