Translate this page into:
Neurogenic bladder following myelopathies: Has it any correlation with neurological and functional recovery?
Address for correspondence: Dr. Anupam Gupta, Department of Neurological Rehabilitation, National Institute of Mental Health and Neuro Sciences, Bangalore - 560 029, Karnataka, India E-mail: drgupta159@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To observe neurogenic bladder pattern in patients with myelopathy by performing urodynamic study (UDS) and to observe whether it has any correlation with functional and neurological recovery.
Patients and Methods:
This prospective study was conducted with 90 patients with myelopathy, both traumatic and non-traumatic (males = 65) in a university tertiary research hospital in India between January 2011 and December 2013. Mean age was 33.5 ± 13.2 years (range 15-65 years), mean duration of injury was 82.63 ± 88.3 days (range 14-365 days) and mean length of stay (LOS) in the rehabilitation unit 42.5 ± 23.3 days (range 14-130 days). The urodynamic study was performed in all the patients to assess the neurogenic bladder pattern. Management was based on the UDS findings. Functional recovery was assessed using Barthel index (BI) scores and spinal cord independence measures (SCIM) scores. Neurological recovery was assessed using ASIA impairment scale (AIS). We tried to correlate neurogenic bladder patterns with recovery.
Results:
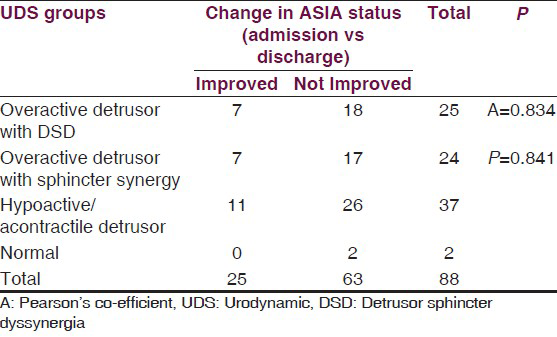
Fifty patients (55.6%) had overactive detrusor with 25 each had detrusor sphincter dyssynergia (DSD) and synergic sphincter. Thirty-eight patients had hypoactive/acontractile detrusor and two had normal studies. No significant correlation observed between neurogenic bladder pattern and change in BI scores (P = 0.696), SCIM scores (P = 0.135) or change in ASIA status (P = 0.841) in the study.
Conclusions:
More than half of the patients with myelopathies had overactive detrusor with or without dyssynergic sphincter according to the urodynamic study. Neurogenic bladder patterns had no significant correlation with functional and neurological recovery in these patients.
Keywords
Functional and neurological outcomes
myelopathies
urodynamic studies
Introduction
Bladder dysfunction is a well-documented part of the clinical presentation of a patient with myelopathies. The term “neurogenic bladder” describes whole spectrum of disorders from acontractile bladder to overactive detrusor to sphincter dysfunctions.[12] The urodynamic study (UDS) to document the presence of lower urinary tract dysfunction and its patterns has brought a significant change in the management of bladder dysfunctions in myelopathy patients. It has also caused significantly decrease incidence of treatment failure and can demonstrate autonomic dysreflexia in these patients.[34] Instrumental UDS generates values for a number of parameters like detrusor pressure, sphincter activity during filling and voiding phase, leak point pressure and detrusor-sphincter dyssynergy which cannot be made out with clinical analysis. UDS is routinely performed in all patients with myelopathies, admitted for in-patient rehabilitation in our department.
The objective of the present study was to observe neurogenic bladder pattern in myelopathy patients by performing UDS and formulate the management strategies based on the results. We also tried to observe if there is any correlation between neurogenic bladder pattern and neurological and functional recovery in these patients.
Patients and Methods
Ninety patients with myelopathies, admitted for in-patient rehabilitation in the department between January 2011 and December 2013, who underwent UDS, were included in the study. Institutional ethics committee approval was taken prior to starting the project. Patients with myelopathies of traumatic or non-traumatic etiology, aged between 15 and 65 years and with duration of insult between 2 weeks and 1 year, admitted for in-patient rehabilitation were included. Patients with recurrent myelopathies, possible intra-cranial involvement like demyelination and patients with cardio-vascular/pulmonary conditions precluding participation in rehabilitation program were excluded. Detailed neurological examination was performed with all the patients. Their neurological status and recovery were recorded both at-admission and at-discharge using ASIA impairment scale (AIS). Functional status and recovery was assessed using the Barthel index (BI) scale and spinal cord independence measures (SCIM) at admission and discharge. UDS findings and subsequent management was also recorded. Pharmacotherapy affecting detrusor or sphincter activity was withdrawn prior to performing UDS.
UDS was performed using multichannel pressure recording technology with Life-Tech. (USA) equipment, Primus. Filling cystometry was performed with the patients in supine position on the urodynamic table. Bladder filling was done with normal saline at medium fill rate. All recordings were made during the procedure (both filling and voiding phase). Sphincter electromyography was performed in all patients to observe sphincter activity and possible synergic/DSD pattern. All data was captured by the software. Analysis of graph and values of relevant pressures was done, final urodynamic diagnosis made and management decided and instituted.
Statistical analysis
Data was entered and analyzed using SPSS software version 15. For correlation of change in functional scores with UDS, the Kruskal-Wallis test was used and for correlation of ASIA status change with UDS, Pearson's test was used. Statistical significance was considered at P < 0.05.
Results
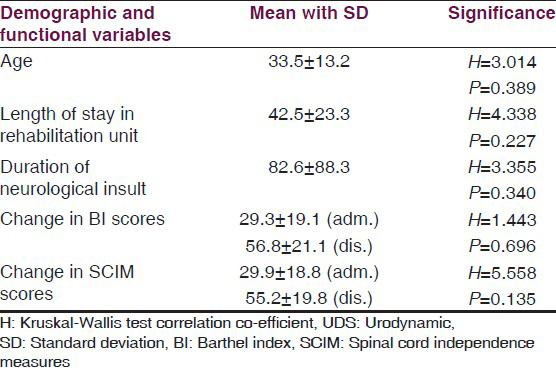
Sixty-five out of 90 patients with myelopathy were males (72.2%). Mean age was 33.5 ± 13.2 years (range 15-65 years), mean duration of neurological insult was 82.6 ± 88.3 days (range 14-365 days) and mean length of stay (LOS) in rehabilitation was 42.5 ± 23.3 days (range 5-130 days). Forty-two patients (46.7%) had traumatic etiology. Among the non-traumatic myelopathy group, 23 patients (25.5%) had transverse myelitis, 10 had primary spinal tumors (11.1%), 7 had prolapsed intervertebral disc lesion (7.7%), 4 had degenerative spinal disease (4.4%), 3 had tuberculosis of the spine (3.3%) and 1 had spinal vascular disease (1.1%).
Considering the topography of myelopathy patients, 18 had cervical level injury, (20%) 40 (44.4%) had dorsal level injury and 32 patients (35.6%) had lumbosacral level of injury.
UDS findings of the patients are presented in Table 1.

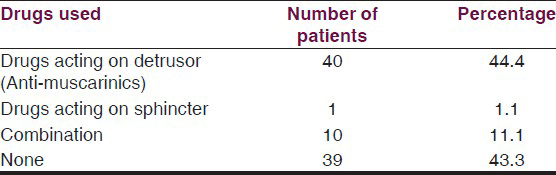
Management of bladder problems was done pharmacologically with anti-muscarinic agents for detrusor hyperactivity and with clean intermittent catheterization in all patients except those with normal studies and no clinical history of urinary complaints. Table 2 illustrates the bladder management strategies adopted. Table 2
On comparison of functional status of patient's at-admission with at-discharge for in-patient rehabilitation program, it was found that there was significant improvement in both BI and SCIM scores in these patients. Mean BI scores were 29.3 ± 19.1 at admission and 56.8 ± 21.1 at discharge (P < 0.001) and mean SCIM scores were 29.9 ± 15.8 at admission and 55.2 ± 19.8 at discharge (P < 0.001).
Correlation of urodynamic findings with age, LOS, functional and neurological improvement is shown in Table 3.
Improvement in ASIA status was considered as any change in ASIA from lower to higher level between admission and discharge (A to B, C, D or B to C or D or C to D). Patients who remained at the same ASIA level at discharge as that at admission were considered to have not improved.
Correlation of UDS with change in ASIA status is shown in Table 4.
Discussion
Neurogenic bladder following myelopathy is more precisely managed after urodynamic studies. High detrusor pressures may lead to upper tract deterioration in due course of time. Protection of the upper urinary tract, maintaining continence and preventing complications like infections and stone formation are the cornerstones of bladder management in myelopathies. Patients whose neurogenic bladders managed on the basis of UDS findings performed periodically would not develop renal damage even as long as 5 years after initial spinal cord damage.[5] Moreover, UDS has been shown to be much more sensitive in predicting renal tract abnormalities at 1 year post spinal cord damage as compared to clinical signs like intact pin prick and bulbocavernosus reflex at 72 hours post injury.[6]
Bladder management plays an important role in spinal cord injured patients. It is well established now that bladder drainage with indwelling catheter is associated with more medical complications, lower social participation and poor satisfaction with life among myelopathy patients.[7] Decatheterization of the patient as soon as possible in the rehabilitation set-up and teaching clean intermittent catheterization/self-catheterization has become the standard norm for managing neurogenic bladders across the world.
Bladder management was determined based on the UDS finding in the present study. In the present study neurogenic bladder patterns showed no co-relation with demographic parameters like age, length of stay in rehabilitation and duration of neurological insult like some earlier studies.[8910]
Fifty out of 90 patients (55.6%) in the present study had overactive detrusor according to UDS. Among these, 25 patients each had synergic/dyssynergic sphincter. Overactive detrusor in such cases is on the expected line.[1112] Thirty-eight patients (42.2%) had hypoactive/acontractile detrusor. As 64.4% of the patients had cervical or dorsal spinal cord damage, the UDS suggests some patients with injuries at these levels had acontractile/hypoactive detrusor. This can be attributed to complete cord injury/ischemic insult or patients still in spinal shock like state. The reverse has also been reported in the literature including in one of our previous studies where patients with cauda equina lesions showing overactive detrusor with or without sphincter dyssynergia.[13] Ganglionic supersensitivity/overactive sacral reflex at sacral spinal center (S2-4) could be the reasons for such detrusor behavior.
When UDS findings were compared with functional recovery using BI and SCIM, no significant correlation was found in the present study. There was no significant correlation between bladder pattern and duration of injury or method of management of urinary tract, unlike a previous study.[14] In a study done on 60 patients with acute SCI, there was no difference in the urodynamic parameters between ambulatory and non-ambulatory individuals. Hence, UDS was recommended strongly in all individuals with acute SCI regardless of functional status.[15] Another study with myelopathy patients observed no relation between level of neurological insult and UDS findings which might mean that expected outcomes of bladder status depending on level of injury may not be borne out when the actual UDS is carried out just like the present study observed.[16]
Previously a study done in the same institution with non-traumatic myelopathy patients found no significant correlation between detrusor activity and level or severity of the lesion.[13] Similarly, follow-up studies by the same group reported that bladder management according to UDS findings among myelopathy patients (traumatic and non-traumatic) had no significant correlation with neurological and functional recovery either during initial admission or when the UDS is repeated in all these patients minimum after 1 year, although significant functional and neurological recovery was observed in these myelopathy patients during follow-up period.[1718] Some factors responsible for this lack of ‘exact’ correlation between anatomic level and neurourologic findings are postulated to be reorganization of neural pathways during recovery process with or without sprouting, incomplete lesions allowing some integration of spinal reflex pathways and undetected multiple injury levels.[19]
In a study done on 36 patients with DSD, it was shown that anal sphincter dilatation reduced external urethral sphincter pressure in ASIA A and B patients significantly more that ASIA C or D.[20] When UDS response was studied in a group of 30 patients with intervertebral disc disease causing spinal cord lesion and neurogenic bladder, there was significant correlation between UDS results and location of disc hernia. (neurological level-cervical, dorsal or lumbar).[21] Thus, ASIA grouping and neurological level might be related to improvement in UDS parameters in selected group of patients.
Conclusions
Urodynamic studies have no significant correlation with functional and neurological improvement in patients with myelopathies according to the present study. UDS must be used as a tool to guide management of neurogenic bladder in this patient population regardless of functional or neurological status.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology. 2013;41:146-55.
- [Google Scholar]
- The various types of neurogenic bladder dysfunction: An update of current therapeutic concepts. Paraplegia. 1990;28:217-29.
- [Google Scholar]
- The role of urodynamics in the management of spinal cord injured patients. Paraplegia. 1984;22:157-61.
- [Google Scholar]
- Assessment of autonomic dysreflexia in patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1997;62:473-7.
- [Google Scholar]
- Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007;26:228-33.
- [Google Scholar]
- Early predictors of bladder recovery and urodynamics after spinal cord injury. Neurourol Urodyn. 1998;17:25-9.
- [Google Scholar]
- Medical and psychosocial complications associated with method of bladder management after traumatic spinal cord injury. Arch Phys Med Rehabil. 2011;92:449-56.
- [Google Scholar]
- Urodynamic management of neurogenic bladder in spinal cord injury. MJAFI. 2009;65:300-4.
- [Google Scholar]
- Comparison of rehabilitation outcomes following vascular-related and traumatic spinal cord injury. J Spinal Cord Med. 2011;34:410-5.
- [Google Scholar]
- Bladder dysfunction in spinal tuberculosis: Clinical, urodynamic and MRI study. Spinal Cord. 2010;48:697-703.
- [Google Scholar]
- Urethral sphincter dyssynergia in spinal cord injury patients. Paraplegia. 1987;25:10-5.
- [Google Scholar]
- Bladder dysfunction in acute transverse myelitis: Magnetic resonance imaging and neurophysiological and urodynamic correlations. J Neurol Neurosurg Psychiatry. 2002;73:154-9.
- [Google Scholar]
- Urodynamic profile of patients with neurogenic bladder following non-traumatic myelopathies. Ann Indian Acad Neurol. 2013;16:42-6.
- [Google Scholar]
- Acute spinal cord injury--do ambulatory patients need urodynamic investigations? J Urol. 2013;189:1369-73.
- [Google Scholar]
- Urodynamic patterns after traumatic spinal cord injury. J Spinal Cord Med 2013 Epub ahead of print
- [Google Scholar]
- Long term assessment of neurogenic bladder following myelopathies by repeat urodynamic study and correlation with neurological and functional recovery. IJPMR. 2012;23:5-9.
- [Google Scholar]
- Urodynamic profile in myelopathies: A follow-up study. Ann Indian Acad Neurol. 2009;12:35-9.
- [Google Scholar]
- Urodynamic patterns after traumatic spinal cord injury. J Spinal Cord Med 2014 Epub ahead of print
- [Google Scholar]
- Urodynamic responses to anal stretch in patients with detrusor sphincter dyssyngergia. Arch Phys Med Rehabil. 2008;89:1748-52.
- [Google Scholar]
- Urodynamic study in the neurogenic bladder dysfunction caused by intervertebral disk hernia. Neurourol Urodyn. 2006;25:446-50.
- [Google Scholar]






