Translate this page into:
Neuro-ophthalmological features due to cerebral toxoplasmosis in an immunocompetent patient: A rare case
*Corresponding author: Putri Permata Valentine, Department of Neurology, Dr. Soetomo General Academic Hospital, Universitas Airlangga Surabaya, Indonesia. putrisiahaan917@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Valentine PP, Ardhi MS. Neuro-ophthalmological features due to cerebral toxoplasmosis in an immunocompetent patient: A rare case. J Neurosci Rural Pract. doi: 10.25259/JNRP_307_2024
Abstract
Toxoplasmosis affects 25–30% of the global population with South America and Asia having the highest known infection rates. The reactivation of toxoplasma gondii cyst can be symptomatic in immunodeficient people but rarely in immunocompetent people. A 38-year-old woman was reported to have experienced restricted visual field since August 2023 with significant weight loss. The result of the HFA test showed hemianopia homonymous sinistra, but normal funduscopic examination. No neurologic deficits were discovered. Visual Evoked Potential (VEP) found bilateral demyelinating lesion on the visual pathway. Multiple lesions were found on Brain MRI. IgM Toxoplasmosis was positive and the result for HIV was negative. Symptoms improved after one month of cotrimoxazole once a day. A follow-up MRI showed decreased edema on the lesions. This report shows immunocompetent people can have atypical manifestation of cerebral toxoplasmosis thas has an excellent outcome on single anti-toxoplamosis treatment after one month. Our diagnosis was based on a detailed history and strong radiological and serological results.
Keywords
Cerebral toxoplasmosis
Hemianopia homonym
Immunocompetent
INTRODUCTION
Toxoplasmosis affects 25–30% of the global population with South America and Asia having the highest known infection rates.[1] Toxoplasmosis can be symptomatic in immunodeficient people but rarely in immunocompetent people.[2] This report described cerebral toxoplasmosis in human immunodeficiency virus (HIV)-negative patients, who had an excellent outcome on single anti-toxoplasmosis treatment.
CASE REPORT
A 38-year-old woman had restricted visual field since August 2023 accompanied by significant weight loss for six months. There was no history of headache, weakness, seizure, or other symptoms. The patient also had a history of a bruise-like rash with a fever. History of previous medical or surgical was denied.
We discovered homonymous hemianopia sinistra on her Humphrey Field Analyzer (HFA), but no abnormalities on her funduscopic abnormality [Figure 1a and b]. Visual evoked potential (VEP) also identified demyelinating lesions affecting both visual pathways [Figure 1c]. Neither focal neurologic deficits were discovered.
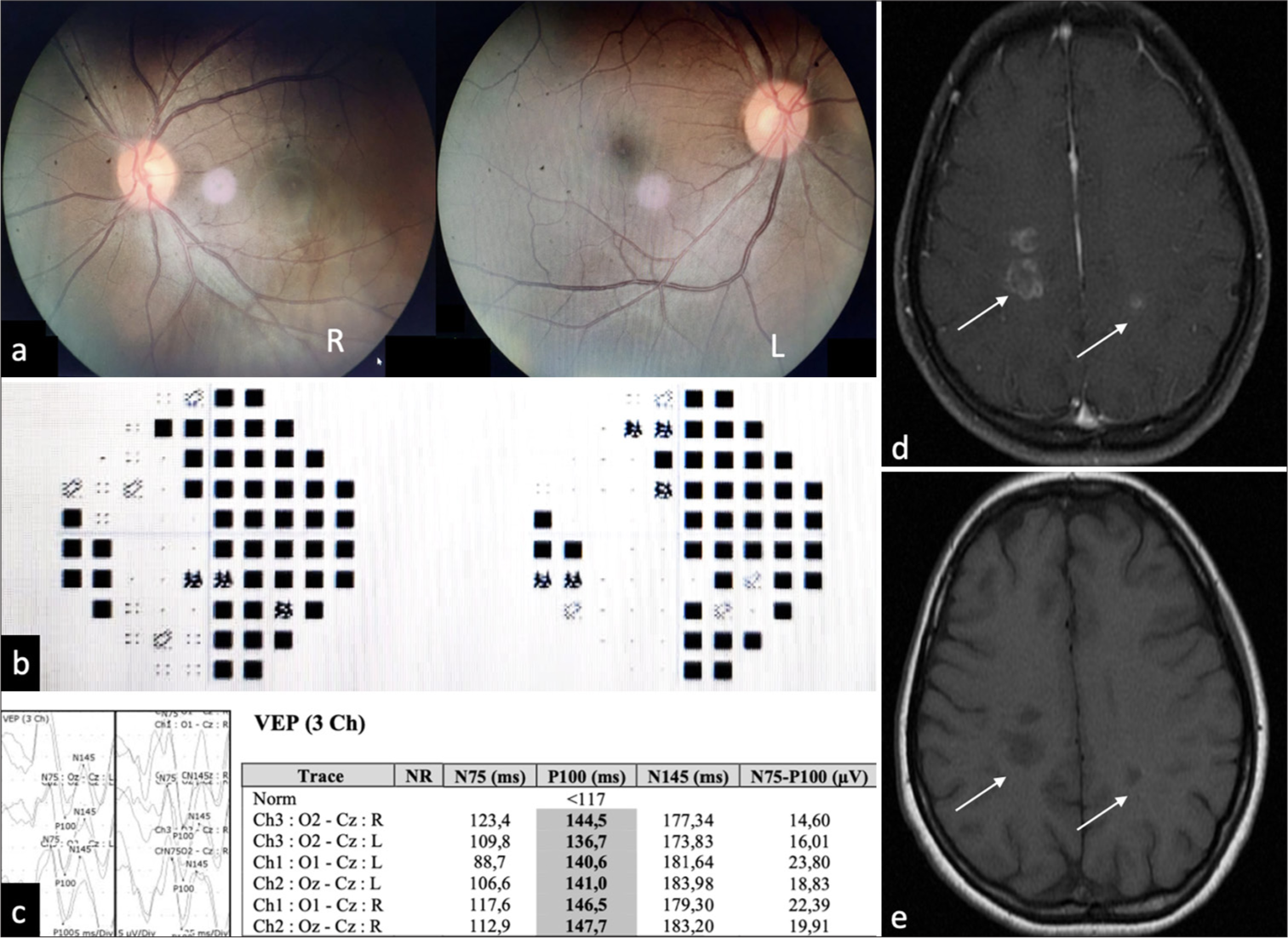
- Diagnostic examination of the patient. (a) Funduscopic examination found no abnormalities. (b) Humphrey field analyzer of this patient showed homonym hemianopia sinistra. (c) Visual evoked potential showed a bilateral demyelinating lesion on the visual pathway. (d) Magnetic resonance imaging (MRI) (September 5, 2023) revealed the appearance of multiple lesions (white arrows) that are hypointense in T1 sequence axial sections. (e) Follow-up MRI revealed the same lesions (white arrows) but decreased perifocal edema.
On magnetic resonance imaging (MRI), multiple lesions with incomplete rim enhancement were detected [Figure 1d]. The laboratory results indicated that the individual tested positive for immunoglobulin (Ig)M reactive toxoplasmosis while negative for HIV. Based on her medical background, radiological imaging, and serology, she was diagnosed with cerebral toxoplasmosis.
The symptoms showed a considerable improvement after administering anti-toxoplasmosis treatment using cotrimoxazole at a dosage of 960 mg once a day for a duration of one month. A follow-up MRI revealed decreased edema on the T1 sequence[Figure 1e].
DISCUSSION
Toxoplasmosis is present in 25–30% of the world’s population, with South America and Asia reporting the greatest infection rates.[1] Toxoplasma gondii, the parasite responsible for cerebral toxoplasmosis, has a remarkable capacity to infiltrate the host and utilize numerous ways to penetrate the blood-brain barrier.[1-3]
In immunocompetent individuals, toxoplasmosis infection rarely causes symptoms, in contrast to immunodeficient persons, reactivation of Toxoplasma gondii cysts can be symptomatic, notably toxoplasma encephalitis.[3] This patient did not have an immunodeficiency, which is unusual.
The primary infection can take up to 24 h and is caused by consuming immature food contaminated with tissue cysts or oocysts. The parasite develops cysts in cerebral and muscular tissues, transitioning from acute to chronic bradyzoites. This persistent parasite can induce immunological problems and reside indefinitely. Stromal cells secrete inflammatory cytokines, activated NK cells attract antigen-presenting cells, and natural killer cells produce interferon-j, prompting dendritic cells to release interleukin-12 and other cytokines. The patient will acquire parasitemia 24–48 h after intake when tachyzoites move from the intestines to other organs.[4]
Clinical manifestations of severe toxoplasmosis in immunocompetent hosts were varied and have been associated with focal lesions or diffuse encephalitis, including fever, chills, flu-like symptoms, anorexia, malaise, night sweats, weight loss, chest pain, cough, dyspnea, hypoxia, pneumonia, pleural effusions, acute respiratory failure, and the need for intensive care unit care. Other symptoms include confusion, agitation, mental status changes, cognitive decline, emotional lability, personality changes, encephalitis, encephalitis, brain-abscess/mass-like lesions, focal neurologic findings, spastic hemiparesis/quadriparesis, pyramidal symptoms, meningitis, meningoencephalitis, rhombencephalitis, cerebellar ataxia, headaches, seizures, central and peripheral neuropathies/nerve palsies, nystagmus, dysarthria, Guillain–Barre syndrome, Miller– Fisher syndrome, hydrocephalus, ventriculitis, and spinal cord lesions.[3,5] Upon examination of this patient, we found hemianopia homonim sinistra confirmed with HFA. VEP also confirmed bilateral demyelinating lesions on the visual pathway [Figure 1c].
Cerebral toxoplasma can be diagnosed with laboratory testing such as the indirect hemagglutination test, the Sabin– Feldman dye test, the indirect antifluorescent substance test, and the enzyme-linked immunosorbent assay test for IgM and IgG antibodies. Anti-toxoplasmosis IgG will rise one to two weeks post-infection, reaching a peak at eight weeks. The titer will gradually decline over the course of a year or two and may persist indefinitely. High levels indicate acute infection or reactivation of a previously dormant illness.[4] The patient tested positive for IgM toxoplasmosis.
Cerebral toxoplasmosis has three clinicopathologic patterns: Neoplasm-like mass effect, diffuse nodular encephalitis, and ependymitis and periventricular necrosis leading to obstructive hydrocephalus.[3,6]
MRI is considered the definitive diagnostic test. Neuroimaging in immunocompromised patients with T. gondii often shows multifocal necrotic, focal hemorrhage from cerebritis or organized abscesses, often ring-enhanced on MRI, in the thalamus, border of substantia grisea-alba, and basal ganglia. This is typically caused by the reactivation of latent infection.[3] These lesions appear dark on T1-weighted images, bright on T2-weighted images and T2 fluid-attenuated inversion recovery, show increased signal on apparent diffusion coefficient and diffusion-weighted imaging, and exhibit incomplete rim enhancement after contrast administration.[1-3,5,7] These lesions correspond to what we found on the patient’s MRI [Figure 1d].
The current treatment only treats acute tachyzoite infection and reactivation.[2] The standard treatment for immunodeficiency is pyrimethamine and sulfadiazine daily for six weeks.[4] Clindamycin is a great alternative to sulfadiazine for those who do not take it well.[3] Antitoxoplasma treatment studies in immunocompetent people differed widely. Most patients had pyrimethamine with sulfadiazine, followed by trimethoprim/sulfamethoxazole. Some patients received non-standard anti-toxoplasma medicines or monotherapies such as spiramycin, azithromycin, erythromycin or clindamycin.[5] The symptoms showed a considerable improvement after anti-toxoplasmosis treatment using cotrimoxazole at a dosage of 960 mg once a day for a duration of one month. A follow-up MRI revealed decreased edema than before [Figure 1e].
CONCLUSION
This report shows that immunocompetent individuals can experience atypical presentation of cerebral toxoplasmosis. The difficulty in diagnosing toxoplasmosis in immunocompetent patients is due to poor clinical suspicion among healthcare providers. In this case, the diagnosis was achieved due to a comprehensive history as well as robust radiological and serological characteristics.
Acknowledgments
The authors would like to thank all who have contributed to the process and completion of this report, including the teaching staff of the Department of Neurology of the Faculty of Medicine, Universitas Airlangga, and Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- HIV-related cerebral toxoplasmosis revisited: Current concepts and controversies of an old disease. J Int Assoc Provide AIDS Care. 2019;18:232595821986731.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral toxoplasmosis in HIV-infected patients over 2015-2018 (a case study of Russia) Epidemiol Infect. 2020;148:e142.
- [CrossRef] [PubMed] [Google Scholar]
- Toxoplasmosis of the central nervous system: Manifestations vary with immune responses. J Neurol Sci. 2021;420:117223.
- [CrossRef] [PubMed] [Google Scholar]
- Toxoplasma gondii and toxoplasmosis In: Scheld WM, Whitley RJ, Marra CM, eds. Infection of the central nervous system (4th ed). Netherlands: Wolters Kluwer; 2004. p. :745-55.
- [Google Scholar]
- Clinical spectrum, radiological findings, and outcomes of severe toxoplasmosis in immunocompetent hosts: A systematic review. Pathogens. 2023;12:543.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral toxoplasmosis in an immunocompetent individual presenting as a solitary space-occupying lesion: A case report. Dubai Med J. 2022;5:256-62.
- [CrossRef] [Google Scholar]
- Development of a risk scoring system for prognostication in HIV-related toxoplasma encephalitis. BMC Infect Dis. 2020;20:923.
- [CrossRef] [PubMed] [Google Scholar]







