Translate this page into:
Metastatic Intracranial Adenoid Cystic Carcinoma with Unknown Primary: Case Report and Review of Literature
Address for correspondence: Dr. Dwarakanath Srinivas, Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India. E-mail: dwarakaneuro@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Adenoid cystic carcinoma (ACC) is an epithelial malignancy comprising approximately 8–10% of all salivary gland tumors. Intracranial ACC without a known primary is an extremely rare pathobiological event. Only 13 cases have been reported in available literature. We report a case of a rare intracranial ACC in a 35-year-old gentleman presenting with features of raised intracranial pressure. Patient had a lesion in the right parieto-occipital lobe and underwent gross total decompression. There was no evidence of any primary after a thorough systemic evaluation. In the postoperative period, he showed a good clinical improvement and was recurrence free at follow-up of 18 months. We also discuss and review the existing relevant literature.
Keywords
Adenoid cystic tumor
intracranial
outcome
primary
surgical decompression
INTRODUCTION
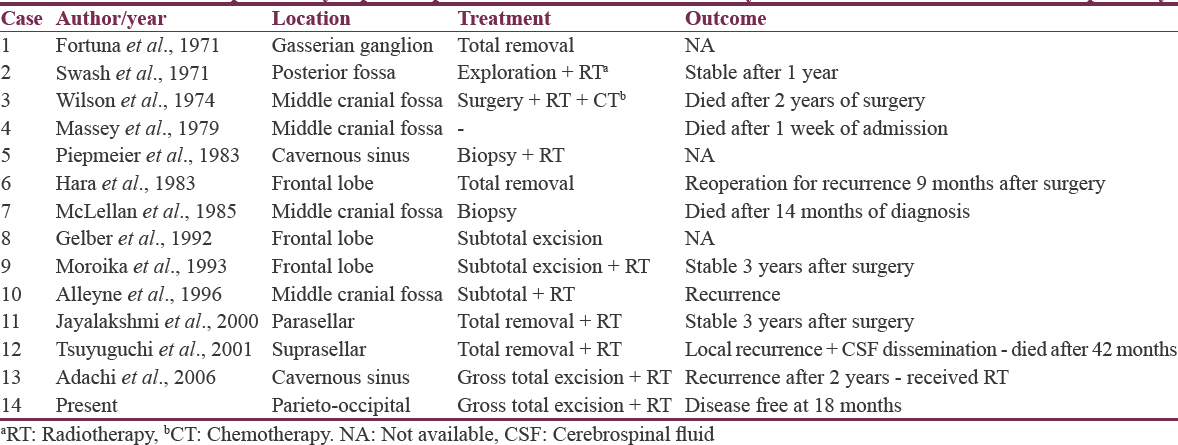
Adenoid cystic carcinoma (ACC) is an epithelial malignancy and constitutes 8–10% of all salivary gland tumors. Most of the reported intracranial ACCs are located at the base of the skull. They arise from the minor or major salivary glands with intracranial spread through perineural, hematogenous, or contiguous extension. The hematogenous spread is the least commonly reported, especially from an unknown primary with only 13 cases having been reported in available literature.[1234]
CASE REPORT
A 35-year-old gentleman presented with clinical features suggestive of raised intracranial pressure lasting for 2 weeks. On clinical examination, he was conscious and oriented. On general physical examination, he had no palpable swelling anywhere else in the body. Neurological examination revealed left-sided homonymous hemianopia with bilateral papilledema. Rest of the examination was within normal limits. Hematological and biochemical parameters were within normal limits.
Cranial computer tomography (CT-plain and contrast) revealed a right parieto-occipital mass lesion. It was isodense on plain scan with an area of hyperdensity situated posteriorly, suggesting calcification. The lesion was enhancing heterogeneously on contrast with significant perilesional edema [Figure 1a]. Magnetic resonance imaging of the brain (plain and contrast) revealed a lesion, which was iso- to hypo-intense on T1-weighted and iso- to hyper-intense on T2-weighted. Lesion was again enhancing heterogeneously on contrast and measured 5.2 cm × 4.4 cm × 4.2 cm [Figure 1b and c]. He underwent right parieto-occipital craniotomy and gross total decompression of the lesion. Peroperatively, the lesion was found to be grayish white, firm to hard, and highly vascular. It had a clear plane of demarcation from the surrounding normal parenchyma.
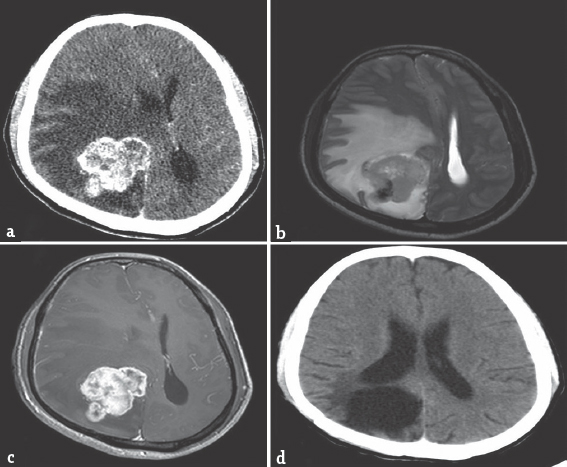
- (a) Cranial computer tomography (postcontrast) revealing a right parieto-occipital mass lesion, enhancing heterogeneously on contrast with significant perilesional edema. (b and c) Magnetic resonance imaging of the brain (plain and contrast) revealing a lesion, isointense to hyperintense on T2-weighted (b) with heterogeneous enhancement on contrast measuring 5.2 cm × 4.4 cm × 4.2 cm (c). (d) Computed tomography (postcontrast) 18 months no residual lesion
Histopathological examination revealed a neoplasm which was sharply demarcated from surrounding normal parenchyma by reactive gliosis [Figure 2a]. The tumor cells were arranged in tubular and cribriform pattern [Figure 2b–d], at places forming ductular structures [Figure 2b] lined by stratified cuboidal cells. Fascicular pattern was not seen. The tubules and acini contained hyaline [Figure 2c] or mucinous material [Figure 2d]. The hyaline material was seen both surrounding the tubules as well as within the tubules. Tubules and acini were highlighted by periodic acid–Schiff stain [Figure 2c, inset]. The tumor was vascular with several hyalinized vessels. The tumor cells expressed cytokeratin (pan cytokeratin) but were negative for chromogranin, thus excluding neuroendocrine tumors. These histological features were characteristic of ACC. No perineural or perivascular spread was evident. Glial fibrillary acidic protein was negative.
![Histopathology reveals a cellular tumor with sharp circumscription from the surrounding brain parenchyma (a). The tumor cells are disposed in tubular and cribriform patterns (b-d) with hyaline (c) material within and around the tumor cells (asterisk, c), highlighted by periodic acid–Schiff stain (c, inset). Focally, bluish myxoid material was seen within the lumen (d) (a: H and E ×10, b-d: H and E ×20, c, inset: Periodic acid–Schiff ×20]](/content/150/2017/8/2/img/JNRP-8-274-g002.png)
- Histopathology reveals a cellular tumor with sharp circumscription from the surrounding brain parenchyma (a). The tumor cells are disposed in tubular and cribriform patterns (b-d) with hyaline (c) material within and around the tumor cells (asterisk, c), highlighted by periodic acid–Schiff stain (c, inset). Focally, bluish myxoid material was seen within the lumen (d) (a: H and E ×10, b-d: H and E ×20, c, inset: Periodic acid–Schiff ×20]
A detailed evaluation including a chest X-ray, ultrasonography of abdomen, and whole-body single photon emission CT was performed to look for the primary site of the lesion. However, no primary site could be identified. Patient received postoperative radiotherapy. He showed good clinical improvement and remains recurrence free at 18 months postoperatively [Figure 1d].
DISCUSSION
ACC, initially called as a “cylindroma” by Billroth, is a malignant tumor arising from secretary cells of the minor and major salivary glands, nasopharynx, lacrimal gland, lung, trachea, mammary gland, and skin. It constitutes 8–10% of all salivary gland tumors, and the frequency of intracranial spread ranges from 4% to 22%.[5] Although ACC is relatively common, intracranial ACC is relatively uncommon. Intracranial spread can occur due to perineural growth, direct extension, and hematogenous spread. The first two modes are the most common, with some authors describing 50% incidence of perineural invasion in ACC. Hematogenous spread is very unusual like the present case, especially in the absence of an unidentifiable primary, with only 13 cases reported in available English literature Table 1. ACC from ectopic intracranial salivary gland tissue has also been reported.[6]
Microscopically, ACCs are characterized by areas of classic cribriform growth pattern, as nests of tumor cells with discrete, rounded, “punched-out” gland-like spaces filled with either eosinophilic or basophilic material. It has diverse histopathological features. The three common types of growth patterns are cribriform (glandular), tubular, and solid. Among these three, the solid pattern has the worst prognosis; however, radiotherapy usually prolongs the survival.[7] In primary ACCs, p53 has shown inconsistent and unreliable expression, but in recurrent tumors, its overexpression has been seen quite often. Another marker expressed in ACC with or without perineural spread is the brain-derived neurotrophic factor.[8] E-cadherin has been shown to have a reduced expression in progressive forms of ACC.
Clinically, intracranial ACCs can present with features of raised intracranial pressure, focal neurological deficits, or a combination of both. Surgical decompression, to reduce the mass effects and provide a pathological confirmation, is the primary treatment for intracranial ACCs followed by radiotherapy. Tumor site, tumor stage, the presence of perineural invasion, and tumor grade are said to be the prognostic factors for ACCs. The survival rates at 5 and 10 years are approximately 50% and 20%, respectively.[7] The role of chemotherapy is still controversial.
CONCLUSION
Primary intracranial ACC is a rare entity, and cell of origin is still unknown. Surgical decompression and postoperative radiotherapy constitute the mainstay of treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Intracranial adenoid cystic carcinoma of suprasellar region. Acta Neurochir (Wien). 2001;143:729-32.
- [Google Scholar]
- Intracranial adenoid cystic carcinoma: case report and review of the literature. Surg Neurol. 1996;45:265-71.
- [Google Scholar]
- Intracranial adenoid cystic carcinoma – A case report. J Neurooncol. 2000;47:47-50.
- [Google Scholar]
- Adenoid cystic carcinoma of the cavernous region. Case report. Neurol Med Chir (Tokyo). 2006;46:358-60.
- [Google Scholar]
- Management and long-term outcome of adenoid cystic carcinoma with intracranial extension: A neurosurgical perspective. Neurosurgery. 1996;38:1105-12.
- [Google Scholar]
- Solitary fibrous tumor of the cerebellopontine angle with salivary gland heterotopia: A unique presentation. Am J Surg Pathol. 2004;28:139-42.
- [Google Scholar]
- Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer. 1994;73:2563-9.
- [Google Scholar]
- Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933-6.
- [Google Scholar]






