Translate this page into:
Melatonin as an add-on anti-seizure medication in children with epilepsy: An open-label randomized controlled trial
*Corresponding author: Amit Kumar Satapathy, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. amitkumar.satapathy@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Muppa V, Mahapatro S, Bhoi S, Satapathy AK, Saini L. Melatonin as an add-on anti-seizure medication in children with epilepsy: An open-label randomized controlled trial. J Neurosci Rural Pract. 2024;15:455-60. doi: 10.25259/JNRP_615_2023
Abstract
Objectives:
The primary objective of this study is to measure the effect of melatonin in decreasing seizure frequency in intervention group as compared to controls in children with drug-resistant epilepsy.
Materials and Methods:
An open-label randomized controlled trial was conducted from July 2020 to June 2022 in children between 2 and 14 years with drug-resistant epilepsy attending Pediatric and Neurology outpatient department and inpatient department. After noting down baseline seizure frequency, children were randomized into the melatonin group and control group. In the melatonin group, add-on melatonin was added to the existing ASM, and in the control group, ASMs were continued. The primary and secondary outcomes were measured after 3 months of follow-up.
Results:
The percentage change in the seizure frequency between both groups at the end of 3 months of follow-up was not statistically significant, but the percentage reduction of seizure frequency was more than 50% in the melatonin group. Melatonin was well tolerated in our children except for 4 (22%) who developed minor adverse effects.
Conclusion:
Add-on melatonin decreases seizure frequency to some extent which was not statistically significant with no major side effects. Further studies are needed to assess add-on melatonin’s long-term effectiveness and safety in children with drug-resistant epilepsy.
Keywords
Epilepsy
Drug-resistant epilepsy
Anti-seizure medication
Melatonin
Seizure
INTRODUCTION
Childhood seizure disorder is the “transient occurrence of signs and symptoms resulting from abnormal, hypersynchronous neuronal activity in the brain.” According to the International League against epilepsy (ILAE, 2017), seizures are classified into focal, generalized, unknown onset, and unclassified based on the mode of seizure onset.[1] Out of 70 million persons with epilepsy worldwide, approximately one-sixth of them reside in India. In a recently published study from India, the overall prevalence was reported to be 3.0–11.9/1,000 population, whereas incidence was 0.2–0.6/1000 population/year.[2] Although no definitive data is available on the prevalence of pediatric epilepsy in India, a study published in Kashmir found it to have a prevalence of 3.74/1000 in boys and 3.13/1000 in girls.[3] Almost 60–70% of the children with epilepsy respond well to available anti-seizure medications (ASMs) as monotherapy if given at adequate doses, but 10– 20% remain refractory and require additional drugs in the form of polytherapy.[4] Hence, there is a need for an add-on ASM with a good safety profile.
Drug-resistant epilepsy is defined as “failure of adequate trials of two tolerated, appropriately chosen and used ASM schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom (all types of seizures) for 12 months, or three times the inter seizure interval before treatment started” (ILAE, 2017).[5] Studies on drug-resistant epilepsy in children from India are scarce. With the availability of newer antiepileptics, identifying this drug-resistant epilepsy is of utmost importance for better management. Despite polytherapy, many of these children continued to be symptomatic. Hence, the need for newer antiepileptics with lesser side effects is warranted. Melatonin is a promising drug with better seizure control and acceptable tolerability, which has been used in limited studies.[6-8]
Melatonin has been widely used in the treatment of sleep disorders. Studies have shown that melatonin increases the quality of life (QOL) in children and adults with epilepsy.[9,10] When compared to normal children, children with epilepsy are shown to have more sleep-related problems. These chronic sleep disorders can have adverse effects on a child’s development. Studies have shown that sleep deprivation exacerbates epilepsy.[11,12] Studies in children with poorly controlled epilepsy showed a decrease in seizure frequency following melatonin treatment when they were no longer sleep-deprived.[13] Melatonin is helpful in the prevention of febrile seizures in children.[14] The anticonvulsant property of melatonin is attributed to its antioxidant, antiexcitotoxic, and free radical scavenging action in the central nervous system providing neuroprotection and hence decreasing the seizure frequency. Blood levels of melatonin are also lower in children with epilepsy compared to normal children.[15] In our proposed study, we tried to measure the efficacy of melatonin as an add-on drug in children with drug-resistant epilepsy.
MATERIALS AND METHODS
This was an open-label randomized controlled trial conducted from July 2020 to December 2022 at a tertiary care center in eastern India. Children with drug-resistant epilepsy (defined by ILAE, 2017), aged 2–14 years, were enrolled in the Pediatric and Neurology outpatients or inpatients. Children with hepatic dysfunction, a history of recent traumatic head injury, status epilepticus, history of allergy to valproate, and melatonin, who were on melatonin products in the last three months were excluded from the study. Ethical approval was obtained from the Institute Ethics Committee (IEC). The trial was registered under Clinical Trials Registry - India before the initiation of the study.
Children attending the Pediatric and Neurology outpatient department (OPD) and inpatient department with drug-resistance epilepsy were included in this study after considering all the inclusion and exclusion criteria. Before enrolling, consent was taken from the parents after explaining the study process in their language. The dose of existing ASMs was optimized at the start of the study. If the child requires adding one more ASM to control seizure frequency, it is added according to existing treatment protocols. Parents were appropriately counseled regarding the maintenance of seizure dairy, which was assessed after one month to know the baseline seizure frequency. Then, the children were randomized.
Randomization
Randomization was done by computer-generated block (in sets of 2 and 4) with an allocation ratio of 1:1 in the study. The “sealed envelope.com,” website was used to generate the randomization list by a third person who was not part of the study.
Intervention
In the control group, ASMs were continued, whereas in the melatonin group, already using ASMs was optimized, plus melatonin as an add-on therapy was added.
Dosage of melatonin
Based on the existing studies in animals and humans considering the comprehensive safety profile of melatonin, the following doses are used in this study: For children <9 years or <30 kg Tab Melatonin (3 mg) 2 tablets (6 mg) was given once daily 1 h before sleep (In children who cannot take tablet directly, the tablet was mixed with the required amount of water and given orally). For children more than nine years or more than 30 kg, a Tab Melatonin (3 mg) and three tablets (9 mg) were given once daily 1 h before sleep.[9]
Outcome measures
Both the control and melatonin groups were followed up by telephonic calls and through WhatsApp weekly for the first month to look for any adverse effects. After that, monthly follow-up was done through pediatric OPD for three months, and seizure dairy was assessed every month for frequency of seizure. A chart containing common side effects such as headache, vomiting, and daytime sleepiness was provided and assessed on monthly follow-ups for three months. Compliance with the medications was ensured by leading questions and by pill counts. If any severe, unusual side effects are noticed on telemedicine follow-up or monthly follow-up, it was planned to discontinue the drug immediately. The same would be reported to the IEC as severe adverse effects. A comparison between the control and melatonin groups for primary and secondary objectives was made at the end of 3 months.
Sample size
Assuming a 25% difference in daily mean seizure frequency reduction (2.0) between the control group (7.8 ± 2) and the experimental group (5.8), with study power of 80% and a- error of 5% and 95% confidence level, we calculated a sample size of 17 in each group. With attrition of 10%, 20 samples in each group were desired.
Statistical analysis
Data was entered in the Microsoft Excel sheet, and Statistical Package for the Social Sciences software (version 21) was used for analysis. Continuous variables were expressed as mean with standard deviation (SD), whereas median with interquartile range was used to express categorical variables. Student’s t-test and Mann–Whitney U-test were used for the analysis of continuous variables, whereas categorical variables were analyzed using the Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Fifty-two children were screened, and 37 were enrolled based on inclusion and exclusion criteria [Figure 1]. Of 37 children, 18 (48.6%) were enrolled in the melatonin group, and the rest, 19 (51.4%), were in the control group. The mean (SD) age of study participants in years in the melatonin group was 6.2 (±3.19) years, and in the control group was 5.6 (±3.73) years, which was not statistically significant. More than 50% of the children had a single type of seizure (GTCS) semiology, whereas the rest had multiple types (GTCS, tonic, and myoclonic). Most children had brief seizure episodes lasting <5 min in both groups. The median age of seizure onset was 24 months in the melatonin group, whereas 36 months in the control group. There was no difference in the duration of seizure onset in either group [Table 1].
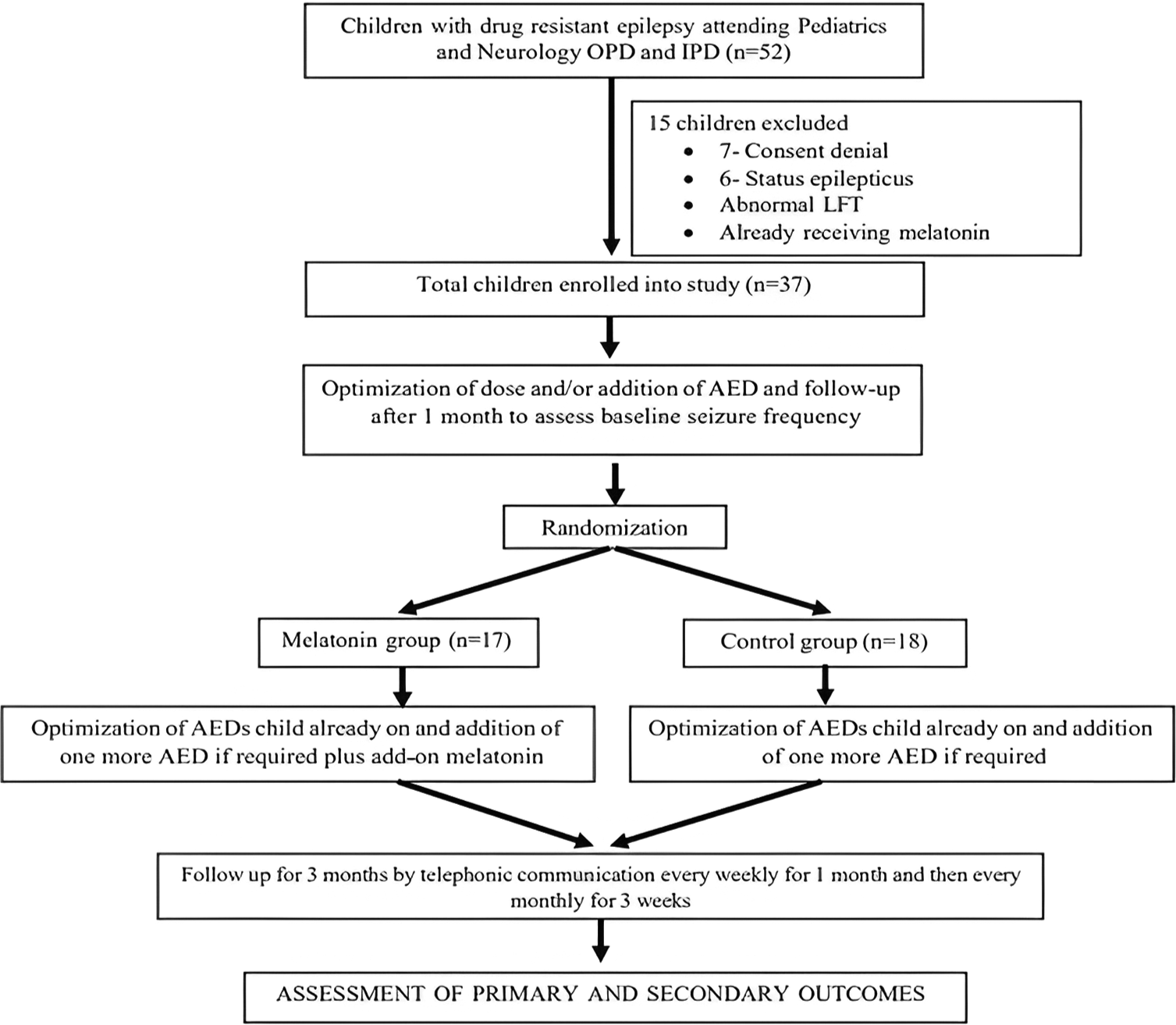
- Study flow chart. OPD: Outpatient department, IPD: Inpatient department, LFT: Liver function test, AED: Antiepileptic drug.
| Parameter | Melatonin group (n=18) | Control group (n=19) | P-value |
|---|---|---|---|
| Age in years (Mean+SD) | 6.22 (3.191) | 5.58 (3.731) | 0.57 |
| Sex | |||
| Male | 12 | 12 | 0.82 |
| Female | 6 | 7 | |
| Type of seizures | |||
| Multiple types | 10 | 7 | 0.25 |
| Single type | 8 | 12 | |
| Duration of seizure episode | |||
| <5 min | 13 | 15 | 0.81 |
| >5 min | 5 | 4 | |
| Age of onset of seizures in months (Median with IQR) | 24 (7–42) | 18 (12–51) | 0.59 |
| Duration of illness in months (Median with IQR) | 36 (17–48) | 36 (12–36) | 0.27 |
| Birth weight (kg) (Mean+SD) | 2.706 (0.469) | 2.663 (0.856) | 0.85 |
| Perinatal events | 9 | 11 | 0.63 |
| Abnormal EEG | 15 | 16 | 0.94 |
| Abnormal MRI | 8 | 11 | 0.65 |
| Number of ASM | |||
| ≤3 drugs | 7 | 12 | |
| ≥3 drugs | 11 | 7 |
SD: Standard deviation, IQR: Interquartile range, EEG: Electroencephalogram, MRI: Magnetic resonance imaging, ASM: Anti-seizure medication
A total of 31 (83%) participants had abnormal electroencephalogram (EEG) findings. There was no significant difference in abnormal EEG findings between the two groups. More than half the participants had abnormal magnetic resonance imagings.
Seizure frequency
Baseline seizure frequency was similar in both groups. Throughout the study, there was a decrease in seizure frequency in both groups. However, this decrease was not statistically significant. When comparing the percentage decrease in seizure frequency between the two groups, the melatonin group showed a reduction of seizure frequency by 51% at the end of the third month whereas the control group also experienced a reduction, but it was only 20%, and this reduction was not statistically significant. At the same time, the number of ASMs in both the melatonin and control groups remained similar throughout the study period [Tables 2 and 3].
| Seizure frequency/per month | Melatonin group | Control group | P-value |
|---|---|---|---|
| Baseline (IQR) | 25 (7–100) | 20 (5–96) | 0.68 |
| 1st Month | 16 (3–76) | 11 (2–60) | 0.34 |
| 2nd Month | 12 (4–45) | 6 (0–40) | 0.90 |
| 3rd Month | 14 (1–50) | 14 (2–22) | 0.39 |
IQR: Interquartile range
| Parameter | Melatonin group (n=18) (%) | Control group (n=19) (%) | P-value |
|---|---|---|---|
| Percentage decrease in seizure frequency at 1 month | 25 | 47 | 0.68 |
| Percentage decrease in seizure frequency at 2 months | 58 | 27 | 0.28 |
| Percentage decrease in seizure frequency at 3 months | 51 | 20 | 0.33 |
Adverse effects in the melatonin group
None of the children had any serious adverse effects. However, four, n = 4 out of 18 (22%) participants had mild side effects in the form of excessive morning sleepiness (n = 2) and vomiting (n = 2), which were relieved after taking anti-emetics.
DISCUSSION
The present study mainly focused on the effect of add-on melatonin in children with drug-resistant epilepsy on seizure frequency. We observed a decrease in the percentage of seizure frequency by 51% in the melatonin group compared to the control though statistically insignificant. The baseline seizure frequency per month before randomization in the melatonin group was 25 (7–100), and in the control group was 20 (5–96). In a previously published double-blind placebo control cross-over pilot study done by Goldberg-stern et al., seizure frequency was documented to be 7.75 per day during the placebo treatment. In contrast, the melatonin group had a seizure frequency of 4.6/day, which was statistically significant. However, the study had a small sample size of 12, of which only ten patients completed the study, and follow-up was only for three weeks.[6]
Similarly, Peled et al., observed a significant clinical improvement in daytime seizure frequency in all children with intractable seizures except one when treated with melatonin and followed up for three months. However, this study was done in only six children.[16] Jain et al. observed a 50% reduction in seizure frequency in two children in their cohort who received melatonin, and eight seizure-free children remained seizure-free during the study. Hence, they did not observe any worsening in seizure frequency. However, children with refractory epilepsy were not enrolled in their study.[7] In most of the studies, seizure control was not the study’s primary objective.
A randomized double-blinded cross-over and placebo-controlled trial by Coppola et al. was done among children with mental retardation age over 12 months, with/without seizures who were diagnosed with sleep disorders where melatonin was compared with placebo. It did not show any significant change in seizure frequency in either group. However, it did not mention an exact number of seizure frequencies in either group.[17] Similarly, another randomized, double-blind, controlled, and cross-over trial by Hancock et al. done in children with tuberous sclerosis with a sleep disorder of age group 18 months–32 years where two different doses of melatonin were compared (5 mg vs. 10 mg). There was no significant change from baseline seizure frequency in either group. Three out of seven participants were seizure-free in this study on follow-up.[18] A Cochrane meta-analysis published in 2016 could not conclude the role of melatonin in decreasing seizure frequency due to a lack of good, quality studies.[19]
In a randomized controlled trial done by Verma et al. in adults with generalized epilepsy where melatonin was used as an add-on drug to valproate, there was a significant decrease in the median number of seizures for eight weeks from three episodes to no episode in the melatonin group as compared to a control group where the decrease was to one episode from three episodes over eight weeks.[10] Melatonin inhibits brain excitability through MT1 and MT2 receptors, which are presumed to be a mechanism for anticonvulsant properties.[20] An animal model has demonstrated the anticonvulsant properties of melatonin.[21] Add-on melatonin is clinically improving seizure control in a study by Gupta et al. However, QOL was considered the primary outcome rather than seizure control in that study.[9]
In our study, 4 out of 18 children in the melatonin group had minor side effects that did not require the stoppage of the drug. Two children had vomiting, relieved with a short course of anti-emetics, and two children had excessive daytime sleepiness in the first two weeks of starting melatonin. The present study’s side effects were similar to previous studies.[22,23] However, none of these adverse drug reactions were observed in children by Coppola et al., Hancock et al., and Brigo et al. Neither melatonin needs to be withdrawn during the study period as in the present study.[17-19]
The present study has limitations of having a small sample size with short-term follow-up and subjective outcomes. We could not repeat the EEG and monitor drug level too.
CONCLUSION
Melatonin had a more substantial effect on reducing seizure frequency compared to the control group, as evidenced by the more than 50% percentage decrease in seizures within the melatonin group without major adverse effects. However, it is important to note that the overall reduction in seizure frequency for both groups was not statistically significant. More research is needed in the future to determine the true effectiveness of melatonin in managing seizures. A large sample size study and a longer follow-up could give us better evidence to establish the findings and to assess the long-term effectiveness and safety of add-on melatonin in children with drug-refractory epilepsy.
Ethical approval
The research/study approved by the Institutional Review Board at AIIMS Bhubaneswar, number IEC/AIIMSBBSR/PG THESIS/2020-21/56, dated June 22, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Commission for classification and terminology. Epilepsia. 2017;58:512-21.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol. 2015;18:263-77.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of epilepsy in school-going children (6-18 years) in Kashmir Valley of North-west India. J Indian Med Assoc. 2009;107:216-8.
- [Google Scholar]
- Predictors of refractory epilepsy in North India: A case-control study. Seizure. 2011;20:779-83.
- [CrossRef] [PubMed] [Google Scholar]
- Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-77.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of melatonin on seizure frequency in intractable epilepsy: A pilot study. J Child Neurol. 2012;27:1524-8.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin improves sleep in children with epilepsy: A randomized, double-blind, crossover study. Sleep Med. 2015;16:637-44.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: Two years' experience. J Pineal Res. 1997;23:97-105.
- [CrossRef] [PubMed] [Google Scholar]
- Add-on melatonin improves quality of life in epileptic children on valproate monotherapy: A randomized, double-blind, placebo-controlled trial. Epilepsy Behav. 2004;5:316-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of add-on melatonin on seizure outcome, neuronal damage, oxidative stress, and quality of life in generalized epilepsy with generalized onset motor seizures in adults: A randomized controlled trial. J Neurosci Res. 2021;99:1618-31.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity between epilepsy and sleep disorders. Epilepsy Res. 2010;90:171-7.
- [CrossRef] [PubMed] [Google Scholar]
- How do children with drug-resistant epilepsy sleep? A clinical and video-PSG study. Epilepsy Behav. 2021;114:107320.
- [CrossRef] [PubMed] [Google Scholar]
- Use of melatonin in the treatment of paediatric sleep disorders. J Pineal Res. 1996;21:193-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between efficacy of melatonin and diazepam for prevention of recurrent simple febrile seizures: A randomized clinical trial. Pediatr Neurol. 2019;101:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in epilepsy and febrile seizures. J Child Neurol. 2010;25:888-91.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin effect on seizures in children with severe neurologic deficit disorders. Epilepsia. 2001;42:1208-10.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in wake? Sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: A double-blind, cross-over, placebo-controlled trial. Brain Dev. 2004;26:373-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of melatonin dosage on sleep disorder in tuberous sclerosis complex. J Child Neurol. 2005;20:78-80.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin as add-on treatment for epilepsy. Cochrane Database Syst Rev. 2016;2016:CD006967.
- [CrossRef] [PubMed] [Google Scholar]
- MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016;56:361-83.
- [CrossRef] [PubMed] [Google Scholar]
- Attenuating effects of melatonin on pilocarpine-induced seizures in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:521-9.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: A systematic review. CNS Drugs. 2019;33:1167-86.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193-200.
- [CrossRef] [PubMed] [Google Scholar]






